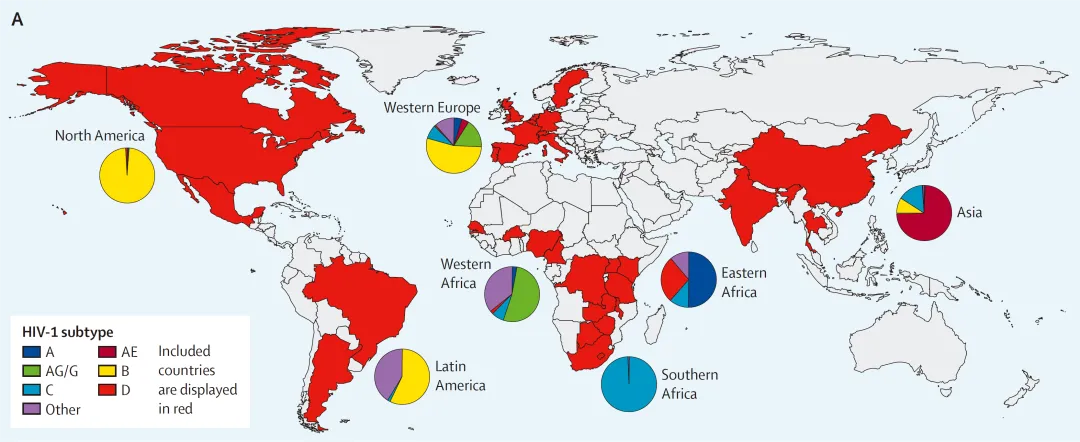
1. Rising Drug Resistance in China: New Guidelines Fuel Innovation in anti-HIV Drug Development
The growing challenge of HIV drug resistance threatens treatment efficacy, accelerates disease progression, increases transmission risks, and significantly limits future therapeutic options—posing serious public health implications. In response, the Center for Drug Evaluation, NMPA (CDE) has released new guidelines targeting these pain points, raising the bar for anti-HIV drug development from the outset and driving the industry toward higher research standards.
2. Aidea Pharma Fulfills Its Misson and Leads Industry Progress
During the critical drafting phase of the CDE’s new Guideline for Clinical Resistance Studies and Data Submission of Anti-HIV Drugs, Aidea Pharma was invited as a key domestic representative of anti-HIV drug developers. The company contributed insights from its extensive experience in resistance research.
Backed by years of commitment to research quality, Aidea Pharma participated in the formulation of these guideline. This has enabled the company to adapt to the new regulations more quickly, having already initiated relevant research prior to the implementation of the new rules.

3. Innovative Therapies Deliver Powerful Solutions Against Resistance
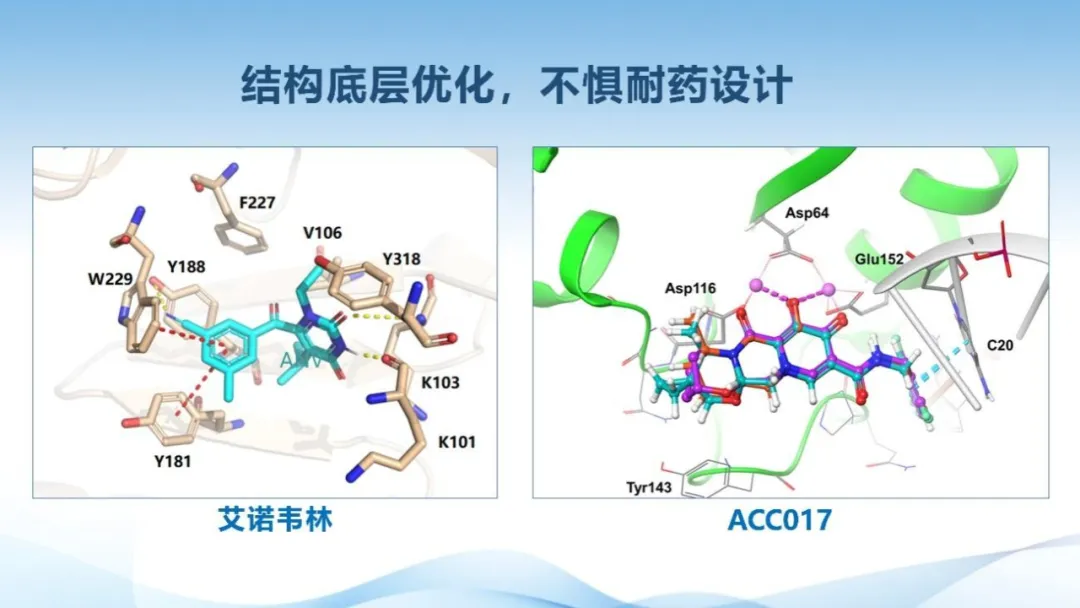
Aidea Pharma's marketed products, Ainuovirine Tablets and Ainuomiti Tablets, have shown strong efficacy and low cross-resistance with existing non-nucleoside reverse transcriptase inhibitors (NNRTIs), offering powerful options to address resistance. Results from the RACER study showed that in People with HIV (PWH) with pre-existing resistance to traditional NNRTIs, Ainuovirine-based regimen achieved virological suppression rates (HIV-RNA < 50 copies/mL) of 95% at Week 24 and 88% at Week 48, both significantly higher than 74% observed in the Efavirenz-based regimen at both time points. Notably, Ainuovirine Tablets’ suppression rates were consistent regardless of baseline resistance mutations—88% in PWH with pre-existing baseline resistance and 89% in wild-type PWH at Week 48.
Aidea Pharma continues to innovate beyond its marketed drugs. Its next-generation integrase inhibitor, ACC017, features a novel chemical structure with high antiviral potency and a strong resistance barrier. As of August 30, 2024, Aidea Pharma has completed the Phase I clinical trial of ACC017. The results confirmed good safety across all dose groups, supporting once-daily dosing.
Currently, ACC017 is undergoing a Phase Ib/IIa clinical trial in treatment-naïve HIV PWH. By the disclosure date of Aidea Pharma’s Q1 2025 report, all subjects had been enrolled and preliminary data indicated that ACC017 showed strong safety and clear antiviral efficacy, achieving rapid viral suppression when used in combination with FTC/TAF. Furthermore, a Phase II clinical study targeting treatment-experienced, drug-resistant PWH is actively underway.
Dr. Fu Heliang, Chairman of Aidea Pharma, stated, "Aidea Pharma has always placed drug resistance research at the core of drug R&D. Starting with its first innovative drug, Ainuovirine Tablets, Aidea Pharma has strictly adhered to international and domestic technical standards and established a comprehensive drug resistance research system. Aidea Pharma will live up to expectations, continue to develop Chinese innovative drugs, and bring benefits to PWH in China and across the globe."

