1. Undiminished Potency, Improved Metabolism: A Dual-Win Treatment Landscape Established
At IAS 2025, five clinical study reports—primarily based on 96-week data from the SPRINT study—systematically evaluated the long-term efficacy and safety of switching from an efavirenz (EFV)-based ART regimen to Ainuomiti Tablets for 96 weeks, and of delayed switching from a TAF-containing regimen (E/C/F/TAF) to Ainuomiti Tablets after 48 weeks of treatment with EFV.
The results showed that in terms of virological suppression, both the group switched to Ainuomiti Tablets (ANV/3TC/TDF) and the group on E/C/F/TAF achieved HIV-1 RNA <50 copies/mL at a rate of 96.6%, demonstrating non-inferiority.
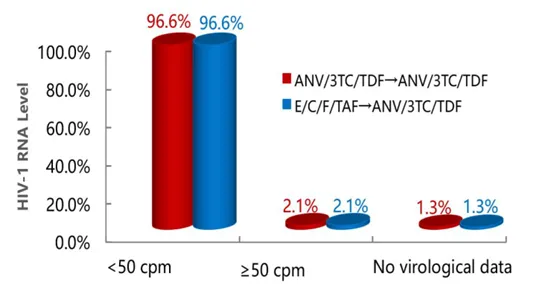
96-week HIV-1 RNA Levels
At the same time, the metabolic benefits of the Ainuomiti Tablets regimen were fully highlighted. Among people living with HIV (PWH) who transitioned from E/C/F/TAF to Ainuomiti Tablets (Delayed Switch Group, DSG), LDL-C decreased significantly by 0.37 mmol/L (P<0.001)
after 96 weeks of treatment, accompanied by decreases in total cholesterol (TC) and triglycerides (TG), as well as a reduction in body weight. Meanwhile, the Immediate Switch Group (ISG) maintained stable lipid metabolism and body weight from weeks 48 to 96.
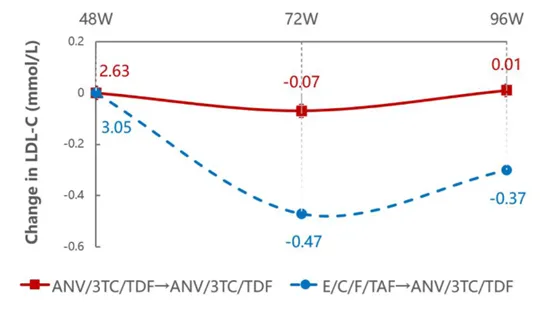
LDL-C Changes from Week 48 to 96
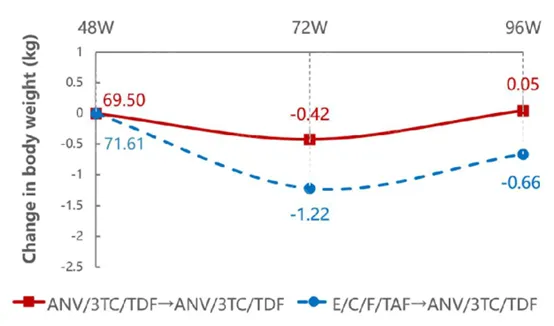
Body Weight Changes from Week 48 to 96
2. Cardiovascular Risk Management: Significant Advantage in ASCVD Prevention
The 96-week SPRINT data also revealed Ainuomiti Tablets's impact on reducing atherosclerotic cardiovascular disease (ASCVD)risk in the Chinese PWH. After switching to Ainuomiti Tablets, both groups showed significant improvements in ASCVD risk-related dyslipidemia stratification.
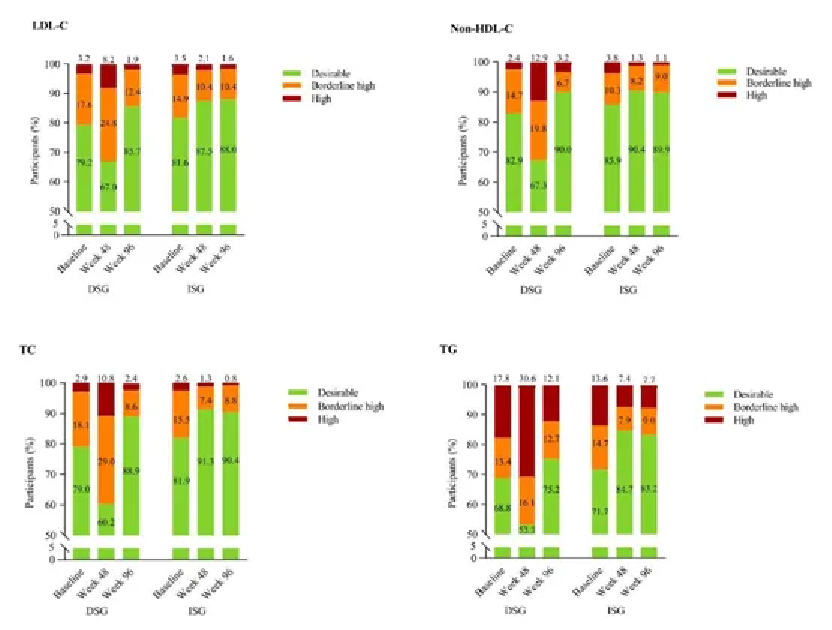
Changes in Dyslipidemia Stratification Related to ASCVD Risk over 96 Weeks
The improvement in lipid profiles with Ainuomiti Tablets is directly associated with reduced ASCVD risk, providing key support for the long-term cardiovascular health management of PWH.
3. Proven Organ Safety: Long-Term Use with Confidence
The 96-week SPRINT study data confirmed the multi-organ safety profile of Ainuomiti Tablets, supporting its use for long-term treatment.
Cardiac Safety: No significant changes in heart rate-corrected QT intervals (QTcF) were observed at week 72 and 96 in either group (only one case showed >60 msec prolongation in the ISG).
Hepatic and Renal Safety:
• Serum creatinine (Scr) remained stable overall, with a slight decrease (-2.7 μmol/L) in the DSG.
• eGFR remained stable in both groups throughout the study.
• Liver enzymes (ALT/AST) remained stable in the ISG, while only minor fluctuations (≤6.9%) were observed in the DSG.
Pancreatic and Muscle Safety: No clinically significant changes were observed in amylase or creatine kinase levels during the study period.
Furthermore, the RACER study and SPRINT subgroup analysis demonstrated that ANV-based ART regimens offer excellent virological suppression and safety in both treatment-naïve and treatment-experienced female PWH. Within 48 weeks of treatment, the ANV-based regimen showed effective viral suppression, with a significantly lower incidence of dizziness compared to EFV-based regimens (16.7% vs. 68.8%), and a markedly lower risk of lipid abnormalities than the E/C/F/TAF regimen (both P < 0.05).
China’s Innovation Reshaping the Long-Term Treatment
Ainuomiti Tablets is not only a potent agent for viral suppression, but also a key contributor to metabolic health. With its notable advantages in weight control and lipid profile improvement, it offers dual clinical protection for PWH—addressing both virological control and metabolic well-being.


