About Aidea
-
33 National 13th Five-Year Plan Science and Technology Major Projects of "Major New Drug Development"
-
22 Transformation of Scientific and Technological Achievements Projects in Jiangsu Province
-
33 Key Technological Innovation Projects in Jiangsu Province
-
4747 Patents
Core competitiveness
-
 Core technologyFresh flow adsorption of urinary protein
Core technologyFresh flow adsorption of urinary protein -
 Strategic programFor solution of major diseases
Strategic programFor solution of major diseases -
 Talent teamFor innovative R&D
Talent teamFor innovative R&D -
 Prospective layoutFor future commercialization
Prospective layoutFor future commercialization
History & Honor



- The company was renamed "Jiangsu Aidea Pharmaceutical Group Co., Ltd."
- The marketing application for Atribond® new drug was approved by the Zambia Food and Drug Administration (ZFDA)
- Phase III clinical trial of the novel integrase inhibitor ACC017 was officially launched
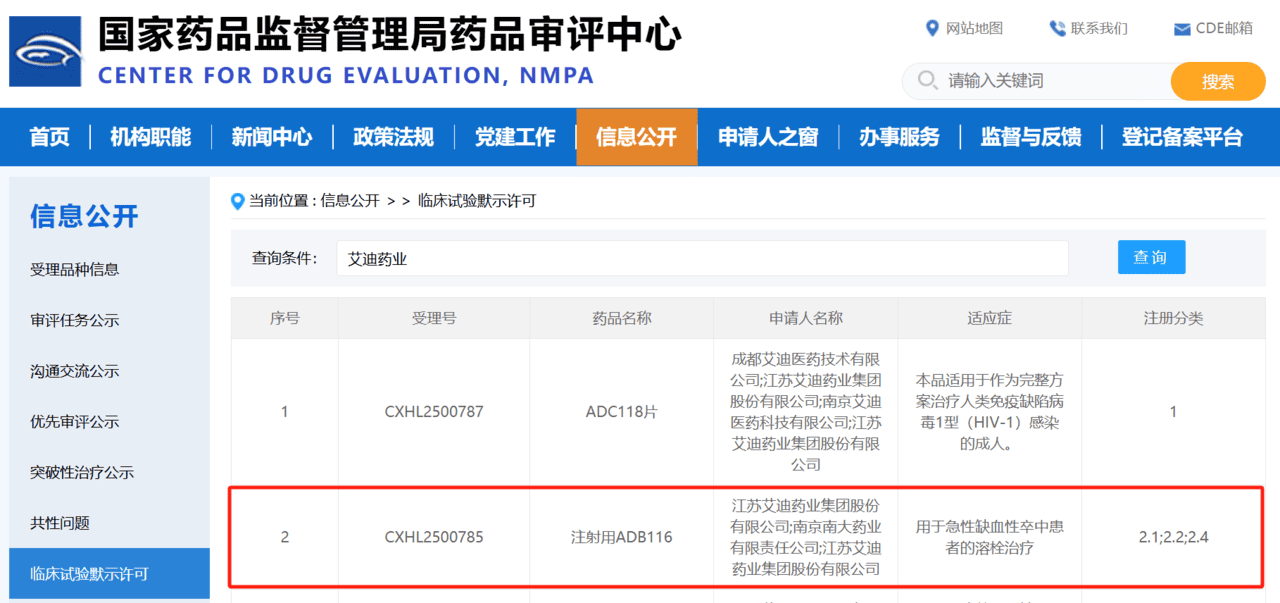
- ADB116 for injection, an improved new drug in the stroke field, was approved for clinical trials
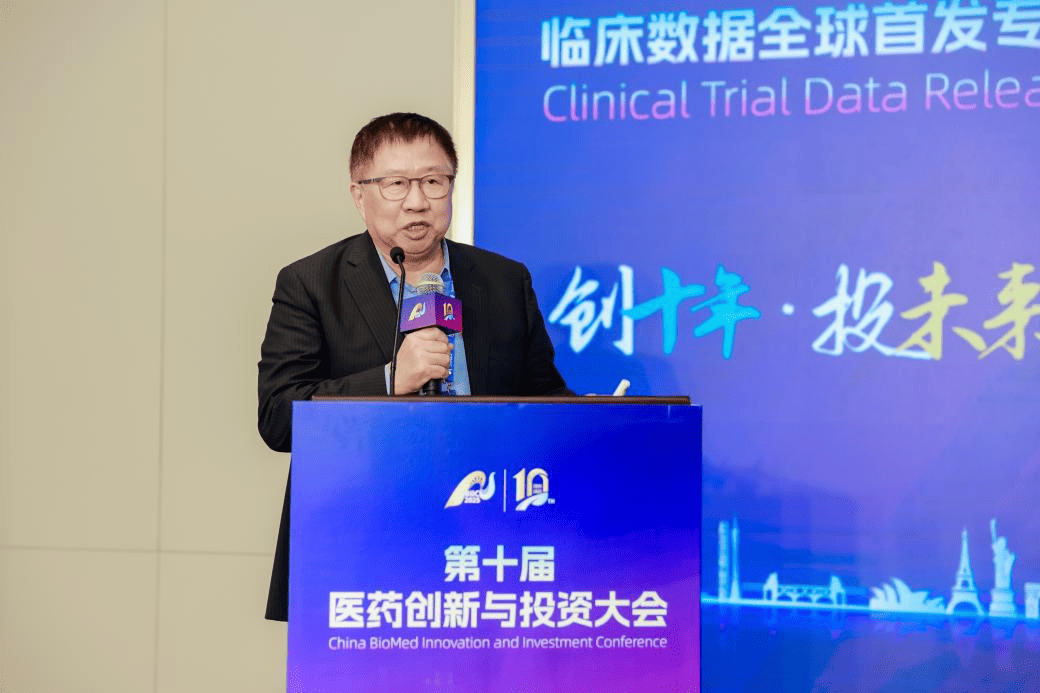
- Dr. Heliang Fu released the Phase II clinical results of Asuptegravir at the 10th Medical Innovation and Investment Conference
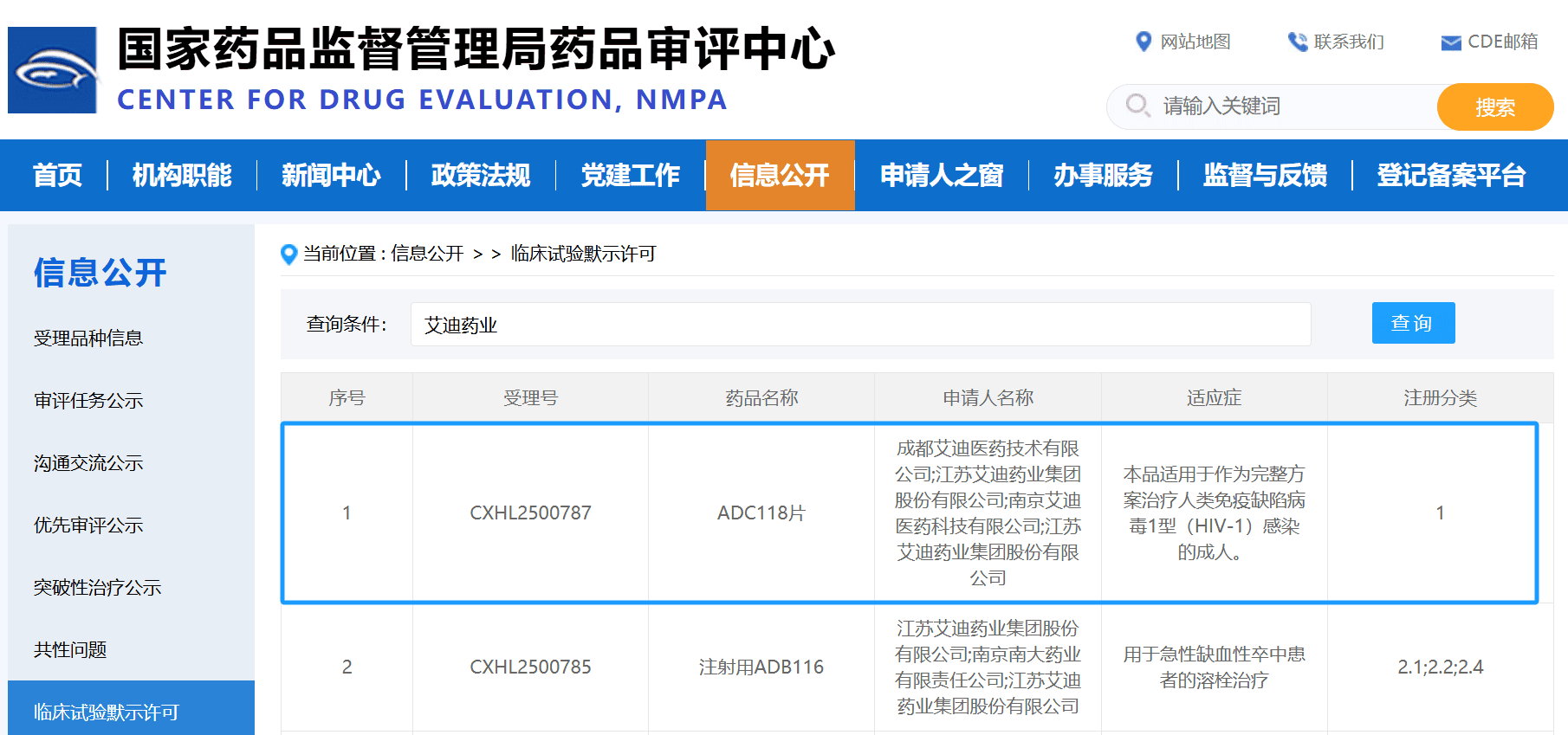
- ADC118 Tablets, a Class 1 new drug in the anti-HIV field, was approved for clinical trials




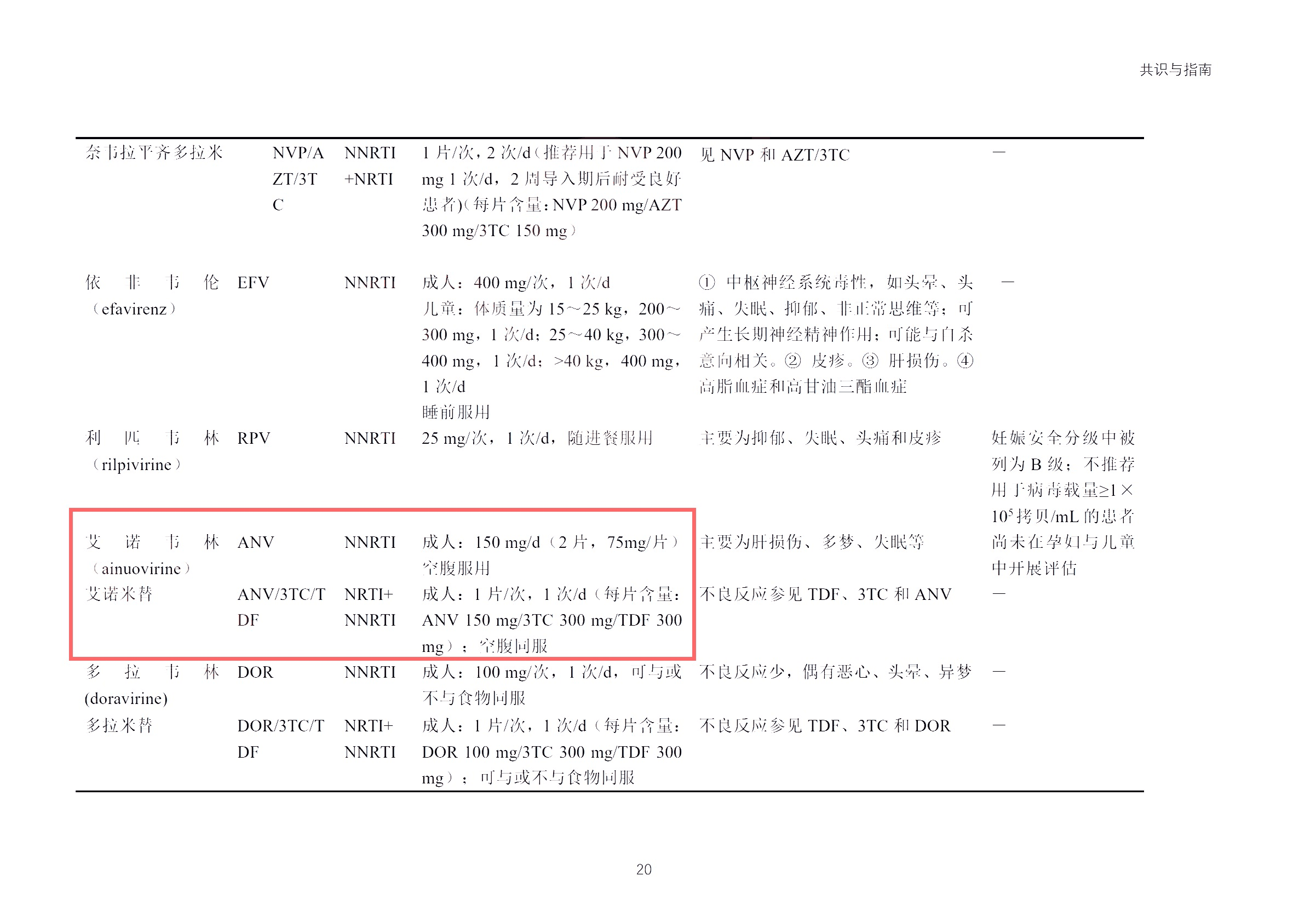




- Acquired control of Nanda Pharmaceutical to achieve "human-derived protein raw material-preparation integration"
- Won the Second Prize of Jiangsu Provincial Science and Technology Award in 2023
- Marketing application for the new indication of Ainuomiti Tablets was approved
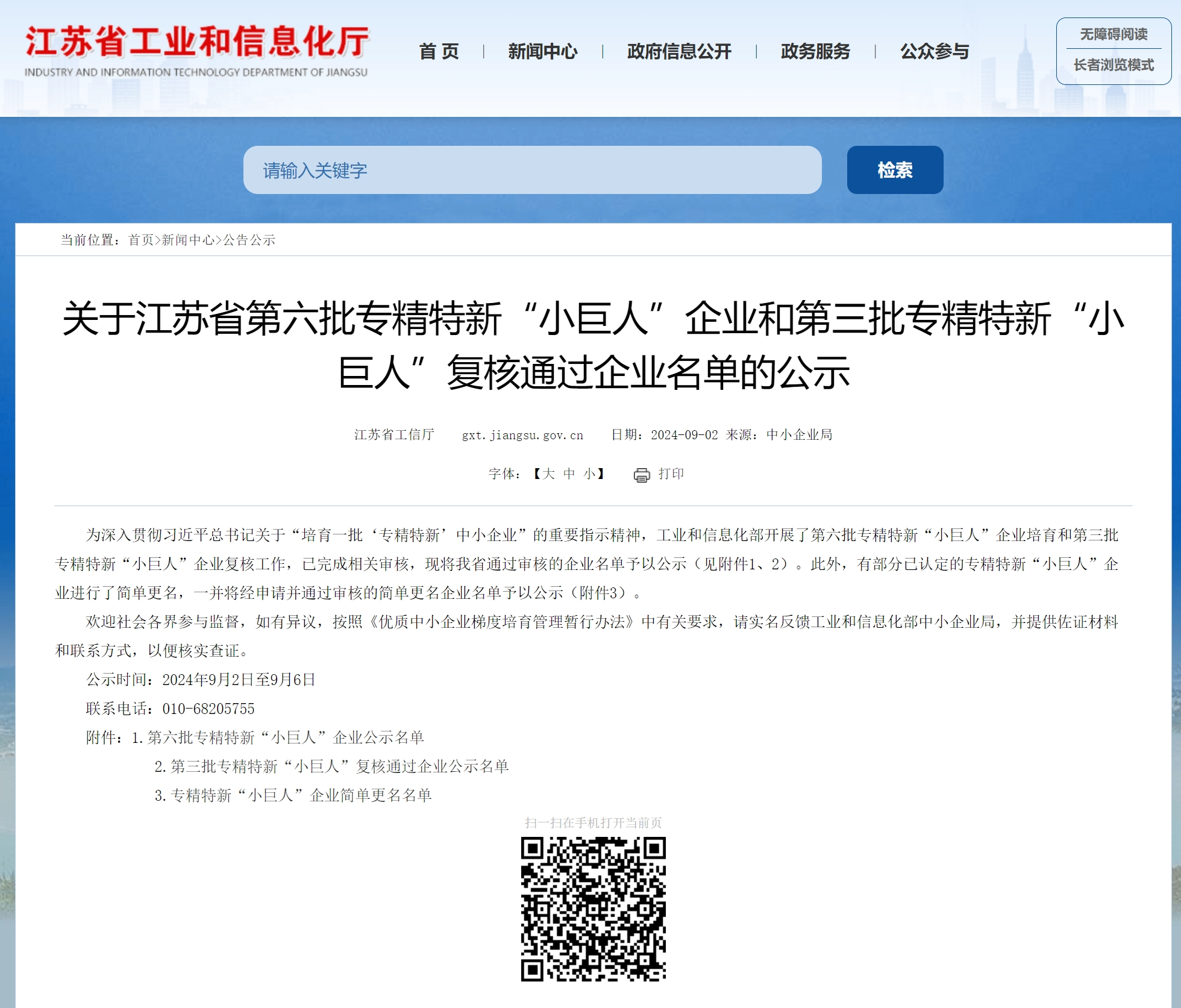
- Selected as a National Specialized, Sophisticated, Unique and New "Little Giant" Enterprise
- Marketing application for Ainuovirine API was approved, and mass production officially started
- Two of the company's anti-AIDS innovative drugs were included in the Chinese Guidelines for the Diagnosis and Treatment of AIDS (2024 Edition)
- Selected into the "2023 China Small Molecule Drug Enterprise Innovation Power TOP30 Ranking"
- The wholly-owned subsidiary "Chengdu Aidea" grandly commenced operations
- Won the title of "2023 Yangzhou Private Enterprise Innovation Top 20"
- Re-certified as a "High-Tech Enterprise"
- Won the title of "2023 Advanced Enterprise in Work Safety"



- The company was selected into the "2022 China Small Molecule Drug Enterprise Innovation Power TOP30 Ranking"
- The company was awarded "Enterprise with the Most Investment Value of the Year" at the 3rd Jiangsu Capital Market Summit
- Co-built the China AIDS Prevention and Control Innovative Drug Research Demonstration Base
- Aitribond® (Ainuomiti/ACC008 Tablets) was included in the National Medical Insurance Catalog, and Ainuovirine/ACC007 Tablets successfully renewed its contract


- Aitribond® (Ainuomiti/ACC008 Tablets) has been approved for marketing
- Signed a Strategic Cooperation Agreement with the Chinese Association of STD & AIDS Prevention and Control (CASAPC)





- Ainuovirine Tablets was included in the national reimbursement drug list. .
- Ainuovirine Tablets was approved for marketing.
- Raw material of Ulinastatin won the title of "Specialized, Fined, Peculiar and Innovative Product of Jiangsu Province”.
- The company received Provincial Special Funds for the Development of Strategic Emerging Industries of Jiangsu in 2021
- The company won the title " Model Unit of Yangzhou 2019-2020".


- Aidea Pharma was successfully listed on the SSE STAR Market (stock abbreviation: Aidea Pharma, stock code: 688488)
- Ainuovirine obtained the Notification of Drug Registration Application Acceptance





- Ulinastatin for injection obtained clinical trial approval
- Aidea Pharma completed the shareholding reorganization and was renamed as "Jiangsu Aidea Pharmaceutical Co., Ltd.".
- ACC008 was included into the national 13th Five-Year Plan Science and Technology Major Projects as"Major New Drug Development".
- ACC010 obtained clinical trial approval.
- Nanjing Accelas Pharmaceutical Technology Co., Ltd. was recognized as one of the Nanjing Antiviral Drug R&D Engineering and Technology Research Centers.



- Ainuovirine compound tablets (ACC008) obtained the approval letter for drug clinical trial.
- Aidea Pharma obtained the pharmaceutical GMP certificate.
- Ainuovirine obtained the provincial special funds for enterprise innovation and achievement transformation in 2018.



- Ainuovirine, a national first class anti-AIDS new drug, was entitled as one of the national 13th Five-Year Plan Science and Technology Major Projects of "Major New Drug Development".
- Ainuovirine, a national first class anti-AIDS new drug, obtained the approval letter for drug clinical trial.
- Aidea Pharma set up a Jiangsu postgraduate workstation.

- Aidea Pharma was renamed as Jiangsu Aidea Pharmaceutical Co., Ltd.



- Aidea Pharma obtained the strategic investments from Jiangsu Hi-Tech Investment Company, Huatai Securities and STARR.
- Aidea Pharma has been recognized as one of the Jiangsu Enterprise Technology Centers.
- A wholly owned acquisition of Yangzhou Xingdou Pharmaceutical, and renamed as Yangzhou Aidea Pharmaceutical Co., Ltd.



- Crude human urinary kallidinogenase was recognized as high-tech products by Jiangsu Province in 2014.
- Aidea Pharma has been recognized as the Human Urine Protein Engineering Technology Research Center of Jiangsu Province
- Aidea Pharma established the enterprise academician workstation.



- The "R&D and Industrialization Project on Fresh Flow Adsorption Technology of Active Protein and Processes for Large-scale Preparation of Ulinastatin and Albumin” obtained the provincial special fund for enterprise innovation and achievement transformation in 2013.
- "Development of the fresh flow adsorption method for industrial-scale production of crude Ulinastatin" won the first prize of the science and technology award.
- Establishment of the subsidiary corporation Nanjing Accelas Pharmaceutical Technology Co., Ltd.
- Raw material of urokinase by fresh flow adsorption was recognized as high-tech products by Jiangsu Province in 2013



- Aidea Pharma was included in the key project list of the Yangzhou Engineering and Technology Research Center in 2012.
- Aidea Pharma was recognized as one of the Yangzhou Enterprise Technology Centers in 2012.
- Crude Ulinastatin containing lower content of vasoactivity substances was recognized as high-tech products by Jiangsu Province in 2012


- The crude urinary albumin was recognized as high-tech products by Jiangsu Province in 2011.
- Aidea Pharma was awarded as "High-tech Enterprise".

- Crude Ulinastatin was recognized as high-tech products by Jiangsu Province in 2010.

- Establishment of Yangzhou Aidea Biotechnology Co., Ltd. (predecessor of Aidea Pharma)
Development program
Our strategy
Meeting needs in the field of HIV/AIDS treatment
Enhancing competitiveness in the field of urinary protein
Our strength
Accelerating drug innovation with rigorous scientific attitude
Generating stable cash flow from urinary protein business
-
 2009-2014Establishment of Aidea
2009-2014Establishment of Aidea -
 2015-2019Growth of Aidea
2015-2019Growth of Aidea -
 2020-2024Fast development of Aidea
2020-2024Fast development of Aidea -
 2025-futureAidea in the leading position
2025-futureAidea in the leading position
Corporate culture
-
 Our vision
Our visionEvolving into a respectable and outstanding pharmaceutical company.
-
 Our value
Our valueIntegrity Accountability Innovation Perseverance
-
 Our mission
Our missionDedicating for better medicine, pursuing greater success.

