At the recently concluded Tokyo APACC 2025 (Asia Pacific AIDS & Co-infections Conference), the new generation non-nucleoside reverse transcriptase inhibitor (NNRTI) Ainuovirine (ANV) and its fixed-dose combination Ainuomiti Tablets (ANV/3TC/TDF) was again highlighted as a key focus. Five real-world clinical studies from frontline China were presented, covering multiple dimensions including virological efficacy, immune reconstitution, metabolic benefits, mental health, and hepatic and renal safety, these studies reaffirmed the clinical value of the regimen.

1. Strong Virological Suppression and Immune Reconstitution: An Optimal Regimen for High Viral Load PWH.
A study targeting newly diagnosed people living with HIV (PWH) with high viral load (≥100,000 cpm) from Southwest China showed that after 48 weeks of treatment with Ainuomiti Tablets, the overall virological suppression rate reached 93.2%, demonstrating excellent virological suppression efficacy. For PWH with a baseline viral load ≥500,000 cpm, the virological suppression rate remained as high as 92.3%. In addition, the average increase in CD4+ T cell count was 129 cells/μL, showing remarkable immune reconstitution in high viral load PWH. (Conference Abstract Poster P62)
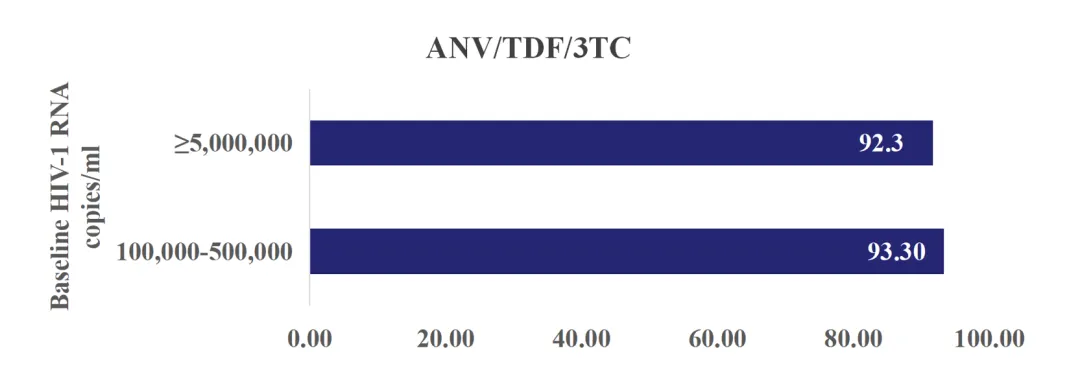
Virological suppression rate at 48 weeks in treatment-naïve PWH with high viral load receiving Ainuomiti
Tablets
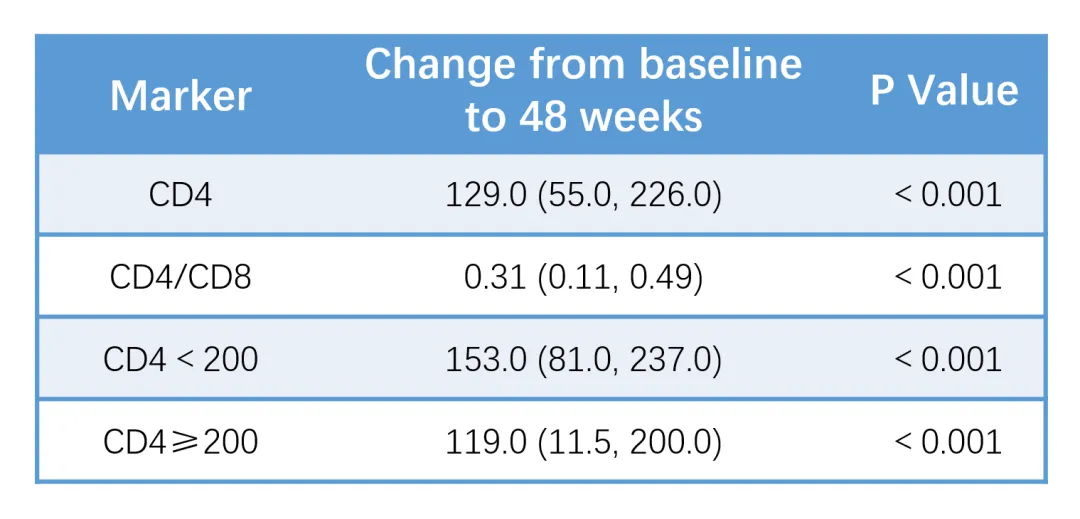
Change in CD4+ T-cell count from baseline after 48 weeks of treatment with Ainuomiti Tablets in PWH
This study indicates that Ainuomiti Tablets has a fast and potent viral suppression ability, while also achieving rapid immune system reconstitution in high viral load PWH.
2. Significant Metabolic Benefits: Supporting Long-Term Patient Management
Multiple studies presented at APACC 2025 collectively validate the metabolic advantages of the ANV-based regimen. Among treatment-experienced PWH who switched to Ainuomiti Tablets, significant reductions in total cholesterol (TC) and triglycerides (TG) were observed after just 12 weeks of treatment, with body weight remaining stable throughout the treatment period. (Poster P295)
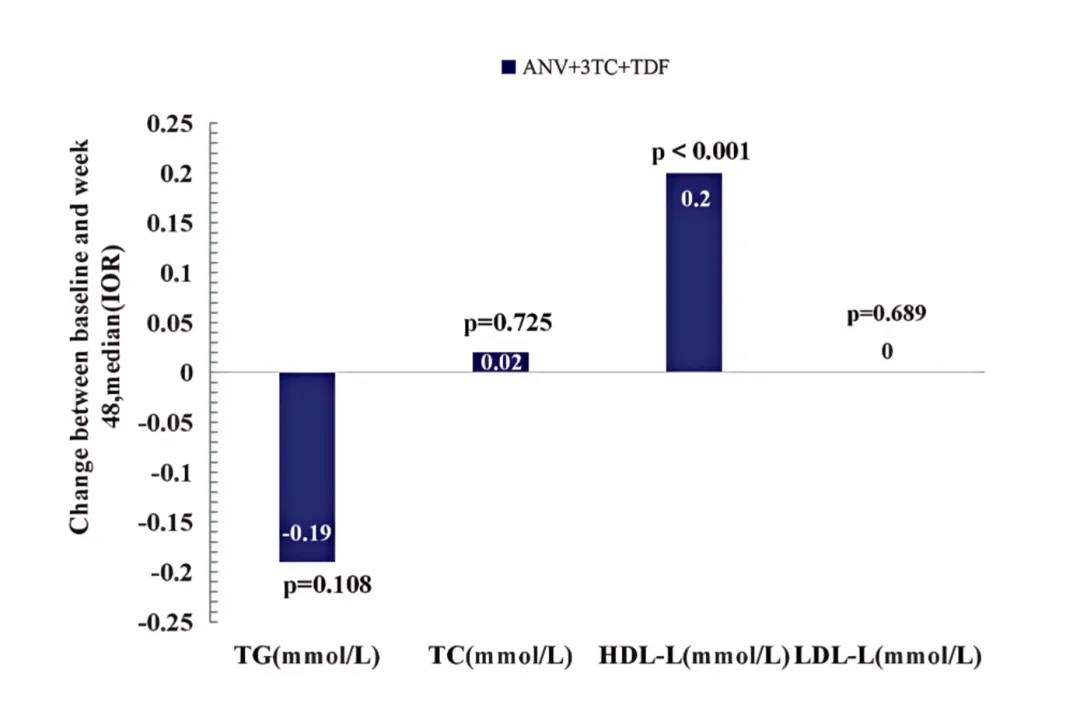
Changes in lipid profiles from baseline after 48 weeks of treatment with Ainuomiti Tablets in PWH
Moreover, long-term treatment with Ainuomiti Tablets showed marked improvement in metabolic indicators. A study found that after 48 weeks of treatment, the protective lipid indicator HDL-C significantly increased (Poster P62). Another study observed that after 18 months of treatment with the ANV-based regimen, TC and TG levels both decreased significantly, and LDL-C levels were significantly reduced in PWH with baseline LDL-C > 3 mmol/L, who required lipid adjustments. (Poster P286)
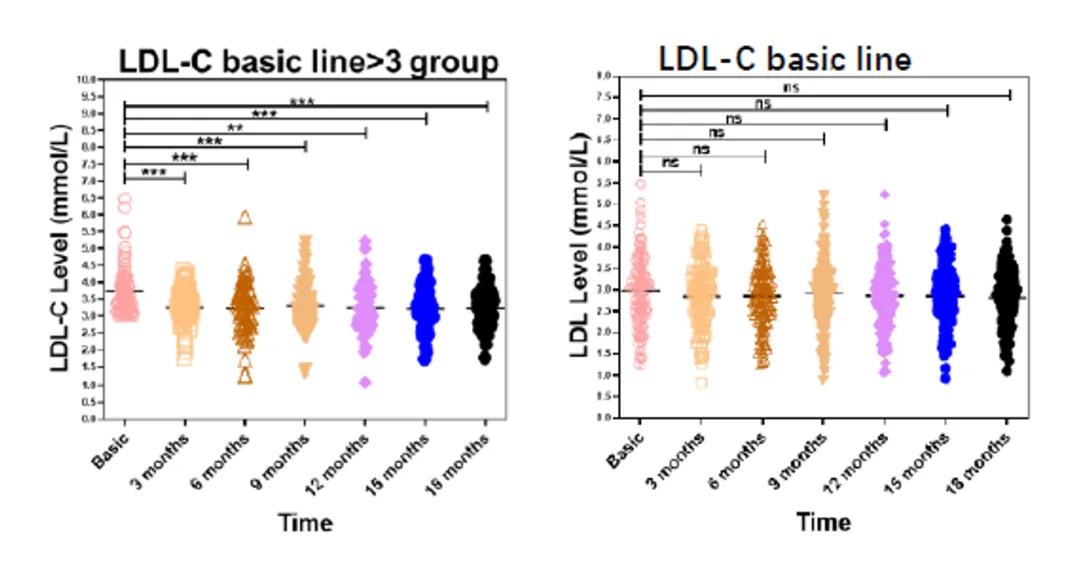
Change in LDL-C from baseline after 18 months of treatment with the ANV-based regimen in PWH
These three studies indicate that after switching to the ANV-based ART regimen, PWH experienced improved lipid metabolism, which aids in long-term adherence to treatment, reduces the risk of cardiovascular disease (CVD), and enhances PWH compliance.
3. Psychological Health Breakthrough: Significant Improvement in Anxiety and Insomnia
In clinical treatment, Psychological and mental side effects are common in ART treatment. At this conference, a new study on the impact of Ainuovirine Tablets on anxiety and sleep quality in PWH was presented. The results showed that after 24 weeks of treatment with ANV, anxiety and sleep quality scores significantly improved (P < 0.05), with a trend toward improvement in depression scores as well. This finding indicates that ANV, as a new generation NNRTI, can offer a new treatment option for PWH suffering from anxiety and insomnia.
4. Hepatic and Renal Safety: Long-Term Real-World Verification
Improvements in hepatic and renal function with the ANV‑based regimen were also observed and analyzed across all studies, each demonstrating a consistent safety profile. After 48 weeks—whether in treatment-naïve patients or patients switched regimens, liver enzyme levels (ALT/AST) remained stable or decreased, and renal function showed no significant fluctuations (Poster P72). In patients with pre-existing hepatic impairment, ALT levels rapid normalization was also observed (Poster P286).
The results of these real‑world studies provide robust evidence supporting the hepatic and renal safety of the ANV‑based regimen, instilling confidence in long‑term clinical use.

Ainuomiti Tablets: Real-World Evidence Building Clinical Trust
Since the launch of Ainuomiti Tablets, Aidea Pharma has been upholding its commitment as a responsible pharmaceutical company, continuously conducting post-market studies to explore the clinical performance of the product in real-world settings.

The five studies on the ANV-based regimen reported at this conference covered PWH from various provinces and regions across China. Through real-world research, these studies systematically validated the multiple advantages of the ANV-based ART regimen in virological efficacy, immune reconstitution, metabolic benefits, psychological health, and hepatic and renal safety. They also added evidence of benefits in complex PWH with comorbidities such as hyperlipidemia and liver dysfunction, providing crucial clinical evidence for the long-term management of HIV/AIDS PWH in China.

