At the recently concluded 13th IAS Conference on HIV Science (IAS 2025), Jiangsu Aidea Pharmaceutical Group Co., Ltd (Aidea Pharma) reported the 96-week data from the Phase III SPRINT study of Aitribond® (Ainuomiti Tablets, ANV/3TC/TDF) in treatment-experienced people living with HIV (PWH) switching from Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Alafenamide (E/C/F/TAF). This report has attracted widespread attention and high recognition from global experts. Well-known international infectious diseases media platform, Touch Infectious Diseases, interviewed Professor Roger Bedimo from the Department of Infectious Diseases at the University of Texas Southwestern Medical Center regarding the latest interpretation of this study. The Clinical Center of Aidea Pharma has compiled and translated the relevant interviews for readers.
IAS 2025 Key Data
The 96-week data from the SPRINT study presented at IAS 2025 in Rwanda, Africa, demonstrated that Aitribond® maintains viral suppression while exhibiting significant metabolic safety advantages. Professor Bedimo stated in the interview: "For PWH with higher cardiovascular metabolic risks or those needing to avoid drug interactions, Aitribond® provides a highly valuable treatment option."

Expert Interview Highlights
Q1:What are the key 96-week efficacy and safety findings from the SPRINT trial, and how do they support switching to ANV/3TC/TDF?
A1: It’s reassuring to see that week 96 efficacy and safety results of the (Switching PLWH to Receive Innovative NNRTI-based Therapy) SPRINT trial confirm the previously published weeks 48 data, indicating a virologic non-inferiority and a superior safety profile for switching to ANV/3TC/TDF compared to elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (EVG/Cobi/FTC/TAF). In week 96 extension study, participants in both arms were switched to ANV/3TC/TDF and followed up for an additional 48 weeks.
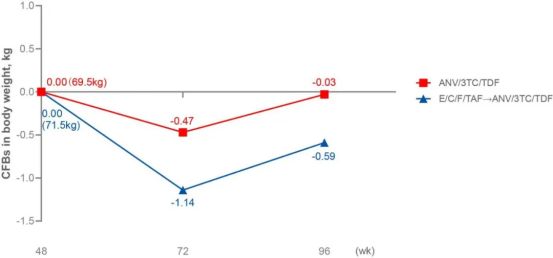
At week 48, participants on ANV/3TC/TDF had lower mean weight gain (1.16 versus 2.05 kg) and lower mean low-density lipoproteins (LDL) increases.
Additional safety results were presented at IAS 2025, including QT prolongation, liver function tests (LFTs) and renal parameters. Also, female participants in the SPRINT trial (n=22) and previously antiretroviral (ARV)-naïve female participants in another trial (RACER; n=34) were pooled in another analysis. At week 48, there were high proportions of participants maintaining virologic suppression.
In the extension study, high rates of virologic suppression were maintained. Also, there were no significant further changes in weight trajectories in either arm, but lipid parameters improved in those who switched to ANV/3TC/TDF at week 96.
Two significant points can be made from the study thus far: 1) as data on ANV is sparse, it is important to see that there are suggestions for the durability of this non-nucleoside reverse-transcriptase inhibitors (NNRTI)-based regimen. 2) While a favourable lipid profile to EVG/Cobi is unsurprising, it’s also reassuring to see that the favourable weight impact was maintained.
Q2: How do the observed changes in weight and LDL-C inform the cardiometabolic profile of this regimen compared with E/C/F/TAF?
Although there is, as of yet, no evidence that this would translate into increased cardiometabolic risk, increased atherogenic lipid profiles on TAF (and EVG/cobi) are a source of some concern. While this is avoided in older NNRTI like efavirenz (EFV), their use has significantly declined due to neuropsychologic complications. So, this might be a reason to consider ANV in a future armamentarium.
The current data suggests that switch to ANV/3TC/TDF yields meaningful cardiometabolic advantages:
l Weight: lower early weight gain compared to EVG/Cobi/FTC/TAF, with stability thereafter, mitigating a known risk of intergrase inhibitor (INSTI) regimens;
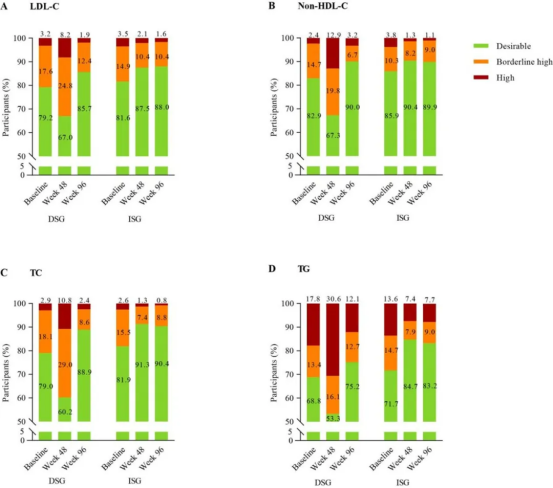
l LDL-C & other lipids: Significant LS mean reductions in LDL-C (−0.39 mmol/L) and total cholesterol (TC) (−0.84 mmol/L) versus increases with EVG/Cobi/FTC/TAF. These changes translate into a more favourable atherosclerotic cardiovascular disease (ASCVD) risk profile, although clinical event data are not yet available.
In short, the combination of weight neutrality and lipid improvements positions ANV/3TC/TDF as a viable alternative for patients at elevated cardiometabolic risk, especially where avoiding CYP3A4 inhibitors is advantageous.
There are essentially four main reasons for antiretroviral optimization, which should be considered when analyzing the potential impact of this regimen:
1) Regimen simplification. This regimen would therefore likely benefit people who are virologically suppressed on complex antiretroviral regimens. At the minimum, it offers them additional options, especially those transitioning from older NNRTI backbones (notably EFV, which accounted for >98% of baseline regimens in SPRINT). It will be more relevant if more data confirms the efficacy in people with some resistance patterns like presence of M184V.
2) Metabolic risk mitigation: Patients with elevated LDL-C, triglycerides (TG), or body mass index (BMI), or those who have already experienced significant INSTI-associated weight gain. Reassuring data on weight neutrality of this regimen would be welcome.
3) Polypharmacy and avoidance of drug-drug interactions. The rapid aging of the HIV population calls for increasing options that would avoid the accumulating non-HIV drugs they will be taking. Comparison to an elvitegravir-containing regimen suggests that should one be willing to avoid CYP 3A4 inhibitors (cobicistat and protease inhibitor containing regimens) this will be a welcome option.
4) Special populations like pregnant people, or children. While data are limited, ANV’s safety profile supports exploration in pregnancy, paediatrics, and women of childbearing potential, with RACER + SPRINT pooled analysis showing good tolerability in women.
Q4: Given these findings, how might this influence future treatment guidelines or switching strategies for virologically suppressed individuals?
We have entered an era where integrase inhibitor-based regimens are given to most people in initial regimens. Given the rationales for antiretroviral therapy optimization cited above, these findings might inform future guidelines on whether to consider this new NNRTI in the optimization options.
Given that current guidelines favor INSTI-based first-line regimens, ANV/3TC/TDF could be positioned in optimization pathways for virologically suppressed individuals in the following scenarios:
l Weight gain or dyslipidemia on INSTIs is problematic;
l Drug-drug interactions with boosters are undesirable;
l NNRTI-experienced but virologically suppressed patients (especially post-EFV) seek a single-tablet alternative.
However, guideline adoption will likely require:
l Comparative data versus modern INSTIs such as bictegravir or dolutegravir;
l Broader resistance data, given ANV’ s similarity to EFV in genetic barrier;
l Real-world cardiometabolic outcomes beyond surrogate markers.
This interview at IAS 2025 conveys a clear message: Aitribond® has demonstrated stable efficacy and good cardiovascular safety. Supported by 96-week data from a multicenter Phase III clinical study, this innovative treatment regimen not only provides personalized treatment options for PWH with different clinical characteristics, but its unique metabolic safety profile also represents a breakthrough in long-term treatment management. With further subsequent research and the accumulation of real-world data, Aitribond® is expected to provide even greater value in clinical practice.