On the frontlines of the global fight against HIV/AIDS, Jiangsu Aidea Pharmaceutical Group Co., Ltd (Aidea Pharma) presented its independently developed new anti-HIV drug Aitribond® (Ainuomiti Tablets, ANV/3TC/TDF) at the 13th IAS Conference on HIV Science (IAS 2025)! This conference is a premier academic event in the global anti-HIV field. Aidea Pharma's participation not only demonstrated the innovative strength of Chinese domestic enterprises but also marked a milestone moment for China's new anti-HIV drugs stepping onto the world stage.

During the multi-day conference, Aidea Pharma released multiple latest research findings on Aitribond®, covering long-term efficacy, weight control, lipid profile improvement, safety, and other dimensions, providing more Chinese solutions for AIDS treatment.
I. Dual Verification of Long-Term Efficacy and Safety
In the 96-week follow-up of the SPRINT study, the Aitribond® treatment regimen maintained a stable viral suppression rate with a good safety profile, and no treatment-related serious adverse events were observed. Whether switching immediately or delaying the switch from E/C/F/TAF to Aitribond®, a high viral suppression rate was maintained, and delayed switching effectively counteracted the weight gain and dyslipidemia caused by TAF in previous treatments.
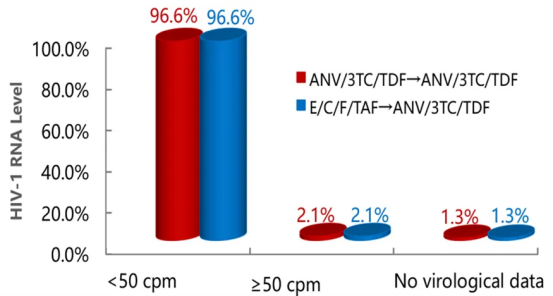
HIV-1 RNA Levels at 96 Weeks of Treatment
II. Dual Control of Weight and Uric Acid, Taking Quality of Life to a New Level
Aitribond® performed excellently in weight and uric acid control. Studies showed that people living with HIV (PWH) continuously treated with Aitribond® remained stable in indicators such as weight, fasting blood glucose, and uric acid levels, while PWH who switched from E/C/F/TAF to Aitribond® showed greater advantages in weight and uric acid control, particularly excelling in long-term treatment.
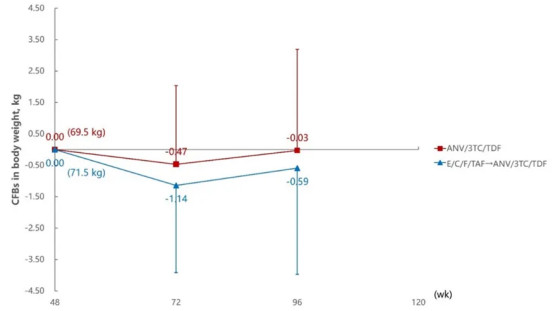
Weight Change Levels at 72 and 96 Weeks Compared to 48 Weeks
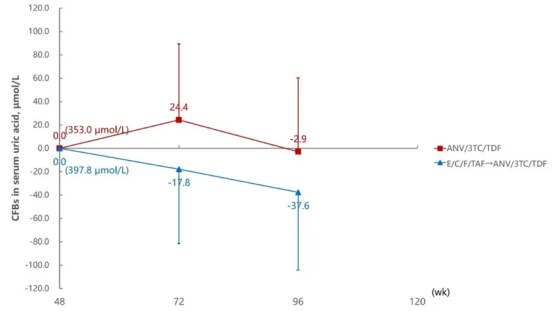
Uric Acid Change Levels at 72 and 96 Weeks Compared to 48 Weeks
III. Improved Lipid Profile, Significantly Reducing Cardiovascular Risk
In the 96-week results of the SPRINT randomized controlled clinical trial, PWH who switched from the E/C/F/TAF regimen to the Aitribond® regimen showed significant improvement in blood lipids, with effective control of ASCVD (Atherosclerotic Cardiovascular Disease)-related dyslipidemia. The 96-week results indicated that Aitribond® not only maintained virological suppression but also improved cardiovascular metabolic risks of PWH .
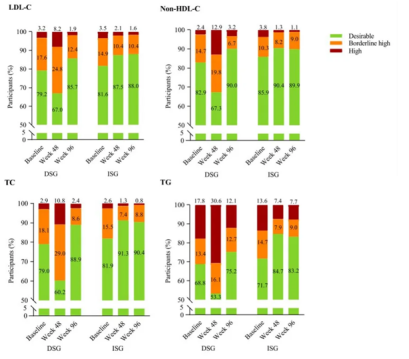
Status of ASCVD Risk-Related Dyslipidemia at 48 and 96 Weeks of Treatment
IV. Safety Assured, Treatment More Reliable
Aitribond® demonstrated excellent safety profiles in key organs such as the heart, liver, and kidneys. Studies showed that after switching to Aitribond® treatment, there were no significant changes in multi-organ related biochemical indicators of PWH, and overall tolerability was good. The study recommended that routine safety laboratory monitoring should be conducted for PWH undergoing regimen switching to ensure treatment safety.
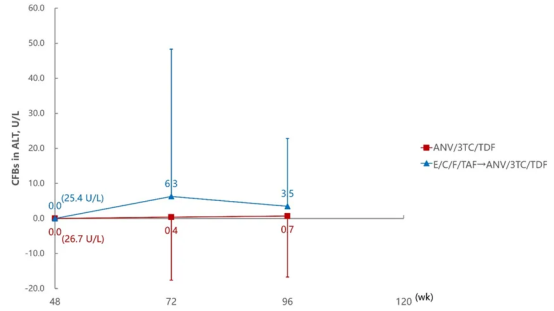
ALT Change Levels at 72 and 96 Weeks Compared to 48 Weeks
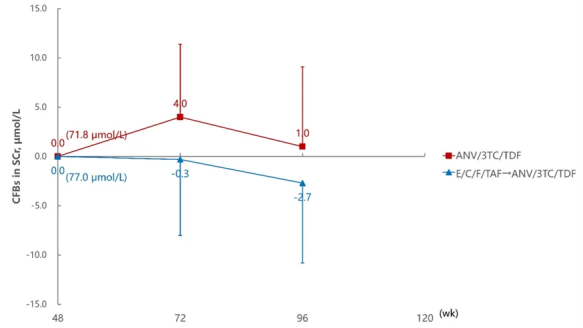
SCR Change Levels at 72 and 96 Weeks Compared to 48 Weeks
The presentation of these results at IAS 2025 marks a solid step forward for Aidea Pharma in the field of HIV treatment and lays a strong foundation for Chinese anti-HIV drugs to go global.
It is worth mentioning that Professor Roger Bedimo from the University of Texas Southwestern Medical Center showed strong interest during the conference in the research results of Aitribond® regarding weight management and lipid improvement. He stated that these data bring new hope for the long-term health management of global PWH, and also lay a solid foundation for future international multi-center collaborative research.