The 10th Asia-Pacific AIDS and Co-Infections Conference (APACC 2025) will be held in Tokyo, Japan from June 12 to 14, 2025. As one of the most influential AIDS academic conferences in the Asia-Pacific region, APACC 2025 will gather top global scientific experts, clinicians, and public health workers to discuss the latest progress in HIV prevention and treatment, especially the challenges faced by young people and possible solutions.

In recent years, the youth population has become a key group for new HIV infections worldwide. These young infected individuals not only face threats from the virus itself but also need to be vigilant against long-term complications such as premature atherosclerotic cardiovascular disease (ASCVD). Against this backdrop, the youth subgroup study of Ainuomiti Tablets (Ainuovirine, Lamivudine and Tenofovir Disoproxil Fumarate Tablets), an innovative anti-HIV/AIDS drug independently developed by Aidea Pharma, has stood out with its breakthrough results and been successfully selected for an oral presentation at APACC 2025. After being invited to the 2024 Hong Kong APACC Conference, Aidea Pharma has once again earned recognition from this prestigious international academic platform.
Professor Fujie Zhang from Beijing Ditan Hospital, Capital Medical University, will share the research results of the "RACER48/SPRINT48 Youth (18-25 years old) Subpopulation" at the conference. This achievement not only fills the gap in the treatment of young people living with HIV ("PWH"), but also brings new treatment options for them.

RACER Study and SPRINT Study are two Phase III clinical trials evaluating the novel non-nucleoside reverse transcriptase inhibitor Ainuovirine (ANV) of Aidea Pharma.
² RACER Study: Research of Efficacy of Ainuovirine- Controlled by Efavirenz-based Regimen
A multicenter, randomized, double-blind, positive parallel control, non-inferior, phase Ⅲ clinical trial, compares the efficacy of the ANV+3TC+TDF regimen with the Efavirenz (EFV) regimen in treatment-naïve PWH.
² SPRINT Study: Switching PWH to Receive Innovative NNRTI-based Therapy
A multicenter, randomized, double-blind, positive parallel control, non-inferior, phase Ⅲ clinical trial, evaluates the feasibility of the ANV/3TC/TDF regimen for treatment-experienced PWH switching population.
Both studies have demonstrated that the ANV-based regimen not only performs excellently in viral suppression and immune recovery but also brings more significant benefits to young PWH, specifically manifested as fewer neuropsychiatric symptoms, less weight gain, and lower lipid levels. These findings provide a new treatment option with both efficacy and safety, as well as sustainability, for PWH, especially the young concerned about long-term metabolic safety and quality of life.
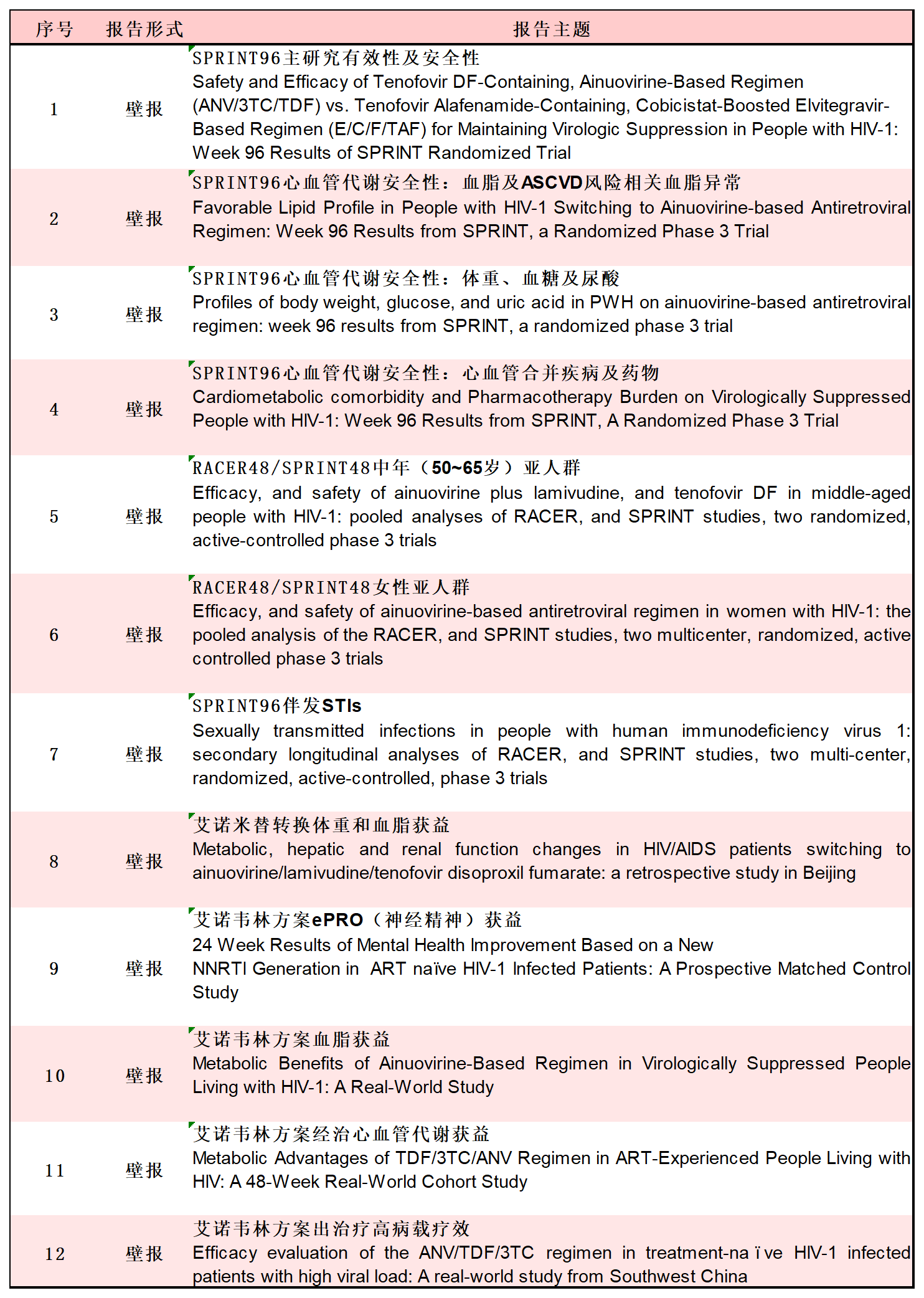
As a leading anti-HIV/AIDS innovator in China, Aidea Pharma has always been committed to providing multiple treatment regimens for PWH. At the upcoming APACC 2025 conference, Ainuomiti Tablets will not only showcase its breakthrough achievements in the treatment of young PWH through a key oral presentation but also demonstrate its clinical value from multiple dimensions through 13 posters. These data cover weight, blood glucose, and uric acid changes, the impact of comorbid cardiovascular diseases and related drugs, neuropsychiatric benefits, and applications in female subgroups and populations with sexually transmitted diseases, among other aspects. Such detailed findings further validate the comprehensive value of Ainuomiti Tablets as a new-generation treatment regimen.
With its advantages of high efficiency and safety, Ainuomiti Tablets is gradually becoming the preferred option for young PWH in the Asia-Pacific region. The achievement presents at APACC 2025 is not only an important milestone for Ainuomiti Tablets in the field of young PWH treatment, but also another proof of China's innovative drugs contributing to the global fight against HIV.


