From April 11th to 14th, the 10th National Academic Conference on HIV/AIDS was successfully held in Zhuhai, Guangdong. Chinese pharmaceutical enterprises are writing a new chapter with their innovative capabilities!
As the first domestic oral single-tablet fixed-dose combination innovative anti-HIV drug, Ainuomiti Tablets, independently developed by Aidea Pharma, has shouldered the mission of benefiting the People with HIV (PWH) with domestically innovative drug since its launch. The 144-week long-term follow-up data announced at this conference not only validates its dual advantages of "efficacy + cardiometabolic protection" but also has redefined China's new standards for anti-HIV treatment with the breakthrough concept of the “The Threefold Path to Health Treatment” (blood lipid management, weight management, and cardiovascular risk management), demonstrating the scientific wisdom and social responsibility of Chinese enterprises.

The SPRINT study of Ainuomiti Tablets is a multi-center, randomized, double-blind, double-dummy, active-controlled phase III trial led by Beijing Ditan Hospital affiliated to Capital Medical University, enrolled 762 virologically suppressed PWH. Subjects received either Ainuomiti Tablets or EVG/Cobi/FTC/TAF regimen from week 0 to week 48, all switched to open-label Ainuomiti Tablets treatment from week 48 to week 96, and then continued follow-up until subjects withdrew from the study.
At this conference, Aidea Pharma shared the 144-week cohort study results of the SPRINT study in virologically suppressed populations switching to Ainuomiti Tablets, further validating the long-term value of Ainuomiti Tablets.
I. Potent Viral Suppression and Sustained Immune Reconstitution
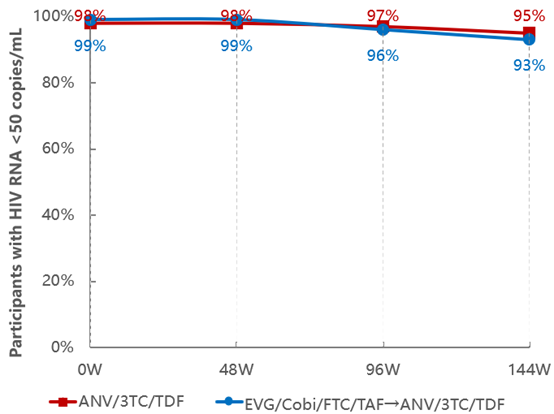
From week 0 to week 144, the treatment adherence and virologic suppression rate of the continuous treatment group with Ainuomiti Tablets remained at a high level (greater than 95%). From week 48 to week 144, after switching from EVG/Cobi/FTC/TAF regimen to Ainuomiti Tablets, the treatment adherence and virologic suppression rate also stayed at a high level (greater than 90%). In addition, the CD4+ cell count continued to increase steadily. The study results show that the sustained virologic suppression rate of Ainuomiti Tablets is superior to that of major imported products [1-3].
Ⅱ. Significant Metabolic Benefits, Reversing INSTIs-Related Weight Gain
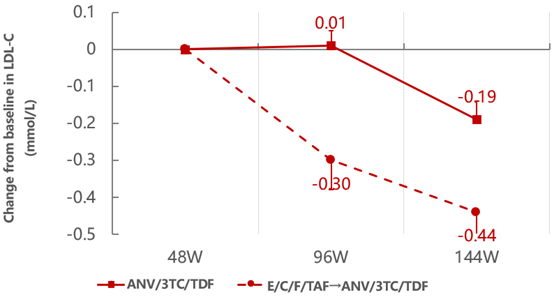
During the 48 - 144 weeks of treatment, the level of "bad cholesterol" LDL-C in the continuous treatment group with Ainuomiti Tablets remained stable and showed a downward trend. After switching from EVG/Cobi/FTC/TAF regimen to Ainuomiti Tablets, the LDL-C level continued to decline significantly.
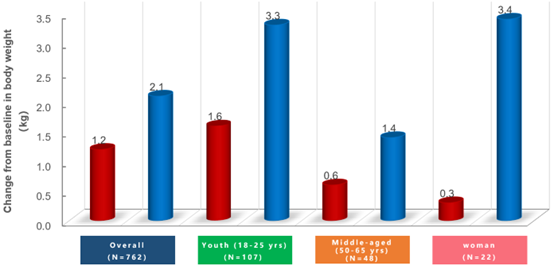
The study results show that from week 0 to week 48, weight gain in the Ainuomiti Tablets group was lower than that in the EVG/Cobi/FTC/TAF group. Switching to Ainuomiti Tablets can reverse weight gain caused by EVG/Cobi/FTC/TAF regimen, with more significant benefits observed in female and young populations.
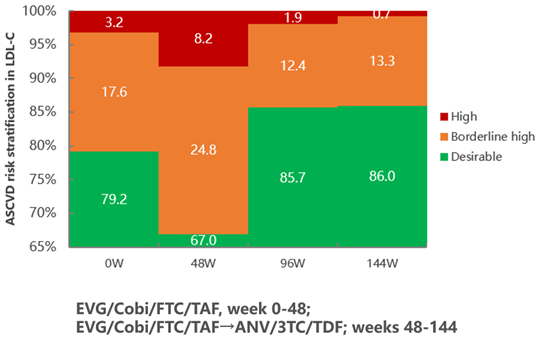
Ⅲ.Reducing the Risk of ASCVD (Atherosclerotic Cardiovascular Disease)
After 144 weeks of continuous treatment with Ainuomiti Tablets, the proportion of high-risk ASCVD populations decreased from 3.5% at baseline to 1.4% (a 60% reduction). In contrast, after switching to EVG/Cobi/FTC/TAF regimen, the proportion of high-risk LDL-C populations surged from 3.2% at baseline to 8.2% (2.6-fold increase). However, after switching to Ainuomiti Tablets, this proportion dropped sharply from 8.2% to 0.7% (a 91.5% reduction), demonstrating significant advantages in cardiovascular risk management.
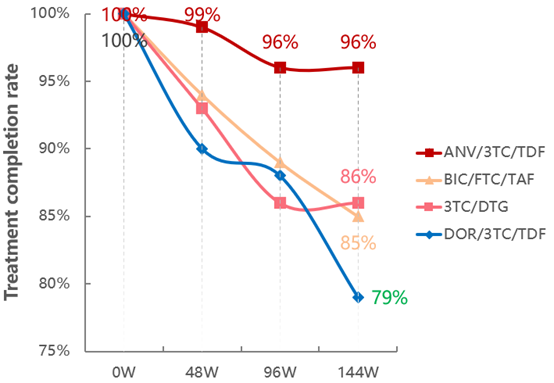
Ⅳ.The Closed Loop of "Efficacy-Safety-Adherence" = The Key to Long-Term Treatment Success
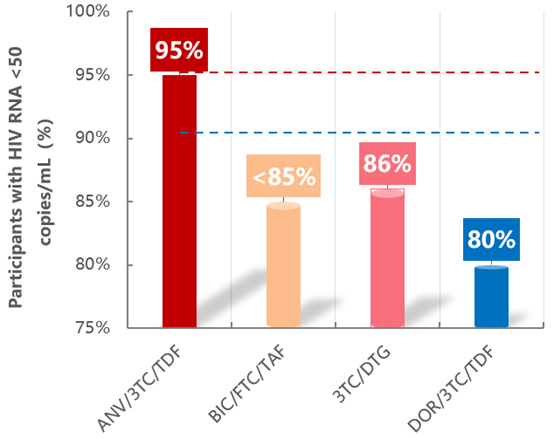
The 144-week data showed that both the treatment completion rate and virologic suppression rate of Ainuomiti Tablets exceed 95%, far surpassing similar imported regimens (both below 90%) [1-3]. This result confirms that its superior safety + proven efficacy = higher adherence among PWH, forming a virtuous cycle and serving as a recognized key to long-term treatment success.
The 144-week study data of Ainuomiti Tablets announced at this conference not only represent a major breakthrough in anti-HIV drugs in China but also signify that national pharmaceutical enterprises have possessed the strength to compete with international giants. With the comprehensive advantages of "potent viral suppression + metabolic protection + high adherence", Ainuomiti Tablets provides PWH with a more friendly and comprehensive treatment option, truly practicing the chronic disease management concept of the "Healthy China 2030" initiative.
References:
1.Brar I, Ruane PJ, Berhe M, Brinson C, Benson P, Henry K, Liu H, Andreatta K, Hindman JT, Ramgopal M. Efficacy and safety of switch to bictegravir/emtricitabine/tenofovir alafenamide from dolutegravir/abacavir/ lamivudine: Results from an open-label extension of a phase 3 randomized, double-blind, multicenter, active-controlled, non-inferiority study. Medicine (Baltimore). 2025;104(8):e41482.
2.Osiyemi O, De Wit S, Ajana F, Bisshop F, Portilla J, Routy JP, Wyen C, Ait-Khaled M, Leone P, Pappa KA, Wang R, Wright J, George N, Wynne B, Aboud M, van Wyk J, Smith KY. Efficacy and Safety of Switching to Dolutegravir/Lamivudine Versus Continuing a Tenofovir Alafenamide-Based 3- or 4-Drug Regimen for Maintenance of Virologic Suppression in Adults Living With Human Immunodeficiency Virus Type 1: Results Through Week 144 From the Phase 3, Noninferiority TANGO Randomized Trial. Clin Infect Dis. 2022;75(6):975-986.
3.Kumar P, Johnson M, Molina JM, Rizzardini G, Cahn P, Bickel M, Wan H, Xu ZJ, Morais C, Sklar P, Greaves W; DRIVE-SHIFT Study Group. Brief Report: Switching to DOR/3TC/TDF Maintains HIV-1 Virologic Suppression Through Week 144 in the DRIVE-SHIFT Trial. J Acquir Immune Defic Syndr. 2021;87(2): 801-805.

