On 24th April, 2023, phase III clinical trial data (Clinical trial ACC007-301) of Ainuovirine tablet, a new oral anti-HIV/AIDS drug (R&D code: ACC007) independently developed by Jiangsu Aidea Pharmaceutical Co., Ltd. (referred to as Aidea Pharma). (SH.688488) in Yangzhou, China, was published in full online in The Lancet Regional Health - Western Pacific, an authoritative international medical journal. This is the first time that China's innovative oral anti-HIV/AIDS drug has been introduced in the top international clinical medicine journal, marking a new stage in China's research on new anti-HIV/AIDS drugs, and contributing new achievements, solutions, and strengths to the global anti-HIV/AIDS cause, especially in the Western Pacific region.
Ainuovirine (ANV) is a new generation of non-nucleoside reverse transcriptase inhibitors (NNRTIs) with excellent pharmacological characteristics such as strong antiviral activity, less cross-resistance, high target selectivity, and less drug-drug interactions. In March 2017, it obtained the clinical trial approval of new chemical drug from the National Medical Products Administration (NMPA), entered the phase I clinical trial in May 2017, and entered the phase III clinical trial in November 2018. Ainuovirine tablet was included in the National Major Scientific and Technological Special Project for "Significant New Drugs Development” during the 13th Five-Year Plan period by the Ministry of Science and Technology of China, and was included in the "Priority Review and Approval" by the Drug Evaluation Center of the NMPA, and later was approved in June 2021. As the first oral anti-HIV/AIDS new drug independently developed in China, Ainuovirine tablet was recommended by the latest anti-HIV/AIDS clinical guidelines in China in the year it was approved, and was successfully included in the List for National Basic Medical Insurance, which has great clinical value and public health significance.
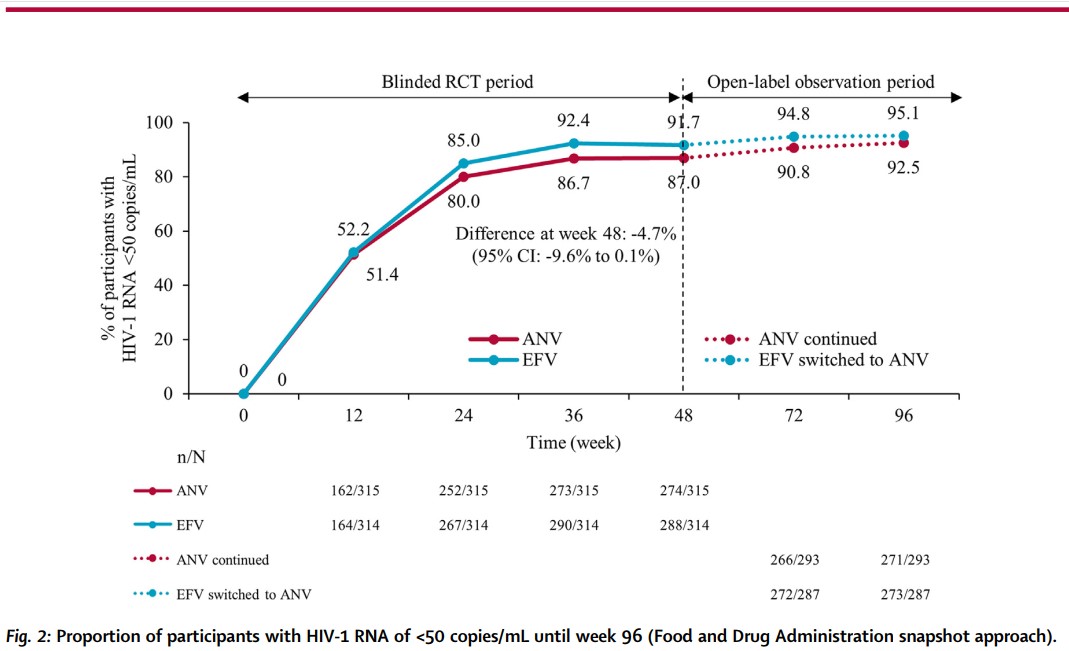
The clinical trial ACC007-301 is a multi-center, randomized, double-blind, double-dummy, positive parallel group, phase III trial led by Professor Hao Wu from Beijing You’an Hospital, Capital Medical University. A total of 7 clinical centers across China participated in the trial (Registration number: ChiCTR1800019041), and it is the first phase III clinical trial of an oral innovative anti-HIV/AIDS drug in China. A total of 630 HIV-1-positive antiretroviral therapy (ART)-naïve adults were randomly assigned in a 1:1 ratio to receive tenofovir disoproxil fumarate and lamivudine (TDF+3TC) in combination with either ANV (ANV group) or efavirenz (EFV group, the most widely used first-line standard treatment in China) once a day for up to 48 weeks in double-blind condition. Subsequently, subjects in both groups received one of the two drug combinations according to their choice until week 96 in an observational study under an open-label setting. The primary endpoint was the proportion of subjects achieving HIV RNA <50 copies/mL at week 48, defined by the US FDA Snapshot algorithm.
At week 48, 274 (87.0%) of 315 subjects in the ANV group and 288 (91.7%) of 314 in the EFV group achieved HIV-1 RNA <50 copies/mL and non-inferiority was established (difference: -4.7%, 95% CI: -9.6 to 0.1%) between the ANV+3TC+TDF vs. EFV+3TC+TDF regimens. Compared with the EFV group, the antiviral effectiveness of ANV was not affected by baseline viral load and immune status CD4 T-cell counts, and the ANV group had a higher elevation of CD4 T-cell counts from baseline than the EFV group. The safety and tolerability of ANV is better than that of EFV, and the adverse drug reaction rates were 67.6% and 91.4% respectively, which dropped to 64.1% when EFV was replaced by ANV; 92% of the subjects chose to continue using or switch to ANV until 96 weeks, while only 0.9% of the subjects chose to continue using or switch to EFV. Compared with EFV, ANV has more obvious advantages in the incidence of side effects such as neuropsychiatric symptoms, dyslipidemia and liver toxicity related to non-nucleoside drugs: dizziness 10.5% vs. 51.0%, abnormal dreams 9.8% vs. 16.2%, dyslipidemia 22.2% vs. 34.4%, transaminase elevation 9.2% vs. 29.0%. When EFV was replaced by ANV, the above adverse reactions were improved to similar levels in the ANV group. The baseline resistance rate in this study was similar to that previously reported for HIV-1-transmitted drug resistance among ART-naïve adults in China, and no participant developed additional drug resistance from week 48 to week 96 of therapy.
Dr. Heliang Fu, the chairman of Aidea Pharma said, "The acceptance and publication of the clinical trial ACC007-301 by the sub-journal of The Lancet marks the international anti-HIV/AIDS academic community's recognition of the scientific nature and compliance of the clinical research on innovative anti-HIV/AIDS drugs in China. This is the result of the long-term joint efforts of China’s anti-HIV/AIDS drug research and development personnels, clinical researchers and the majority of subjects. It also encourages Aidea Pharma to continue to deepen its anti-HIV/AIDS cause, and develop better anti-HIV/AIDS innovative drugs including long-acting drugs and functional cure drugs for the HIV/AIDS infectors in China and the world. This is also a solemn commitment made by Aidea Pharma for the cause of anti-HIV/AIDS."
The title of the paper is "Efficacy and safety of ainuovirine versus efavirenz combination therapies with lamivudine/tenofovir disoproxil fumarate for medication of treatment-naïve HIV-1-positive adults: week 48 results of a randomized controlled phase 3 clinical trial followed by an open-label Setting until week 96". The seven clinical research centers across China include Beijing You’an Hospital, Capital Medical University, Beijing Ditan Hospital, Capital Medical University, The First Hospital of Changsha, Chongqing Public Health Medical Center, The Eighth People’s Hospital, Guangzhou Medical University, The Second Hospital of Nanjing, Nanjing University of Chinese Medicine, and Infectious Disease Hospital of Henan Province (The Sixth People's Hospital of Zhengzhou). The first authors of this article are: Bin Su, Guiju Gao, Min Wang, Yanqiu Lu, Linghua Li, Chen Chen, Yuanyuan Chen. The corresponding authors are: Qingxia Zhao, Hongxia Wei, Weiping Cai, Yaokai Chen, Fujie Zhang, and Hao Wu (the main responsible person).
Link to the full text of the paper:
https://www.thelancet.com/journals/lanwpc/article/PIIS2666-6065(23)00087-1/fulltext
https://www.sciencedirect.com/science/article/pii/S2666606523000871

