Aitribond® achieves a new milestone in global academic publications: SPRINT 96-Week Data Published in BMC Medicine (5-Year Impact Factor: 9.4). Previously, the SPRINT 48-week study was published in a Lancet sub-journal (Impact Factor: 8.1).
On October 11, Aidea Pharma (688488.SH) announced that the full results of the pivotal Phase III clinical study (SPRINT 96-week extension trial in treatment-experienced switching populations) for its self-developed, China's first "three-in-one" anti-HIV single-tablet regimen innovative drug and new-generation NNRTI-based combination, Ainuomiti (Aitribond®, ALT), have been published in BMC Medicine, a flagship medical journal under the global academic publishing giant Springer Nature. The journal is recognized as a top-tier JCR and Chinese Academy of Sciences Category 1 journal, with a five-year impact factor of 9.4.

Over the 96-week study period, the SPRINT study delivered an impressive long-term report: people living with HIV (PWH) not only demonstrated high treatment adherence and excellent virological suppression but also showed cardiometabolic safety benefits with the potential to influence clinical guidelines [1].
Furthermore, the impact metrics of this publication have surpassed those of imported comparable products.
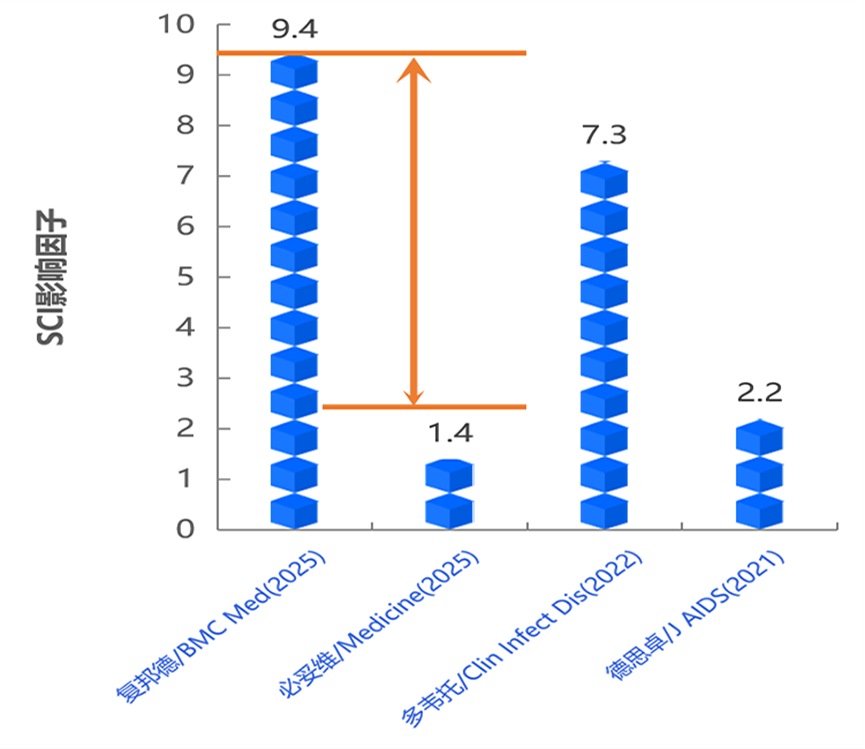
*Compiled based on publicly available academic publications of the relevant studies.
1. Overall Efficacy and Safety: Sustained Performance over 96 Weeks
The SPRINT study was a multicenter, randomized, double-blind, active-controlled, parallel-group Phase III trial. The study consisted of two phases: In the first phase, 762 PWH previously virologically suppressed on an NNRTI-based regimen were randomized to receive 48 weeks of double-blind Ainuomiti Tablets (N=381) or Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Alafenamide (E/C/F/TAF) (N=381). In the second phase, all PWH continued receiving open-label Ainuomiti Tablets treatment until Week 96. Following completion of the SPRINT study, eligible PWH entered a real-world extension study.
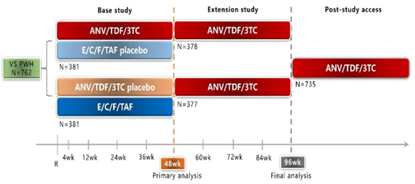
*Diagram of SPRINT study design
· Long-Term Benefit of Sustained Virological Suppression:
At Week 96, the primary endpoint (HIV RNA ≥50 copies/mL) was 3.4% in both the Ainuomiti Tablets continuous treatment group (Immediate Switch Group [ISG], N=381) and the E/C/F/TAF to Ainuomiti Tablets switch group (Delayed Switch Group [DSG], N=377) (FDA Snapshot algorithm, ITT-EXP analysis). These results met the pre-specified non-inferiority margin of 5% for the primary efficacy endpoint.
The virological suppression rates reached 97% in both groups, exceeding those reported in 96-week clinical trials of comparable marketed imported regimens (Bictegravir/Emtricitabine/Tenofovir Alafenamide [BIC/FTC/TAF]: 89%; Dolutegravir/Lamivudine [DTG/3TC]: 86%; Doravirine/Lamivudine/Tenofovir Disoproxil Fumarate [DOR/3TC/TDF]: 84%) [2-4].
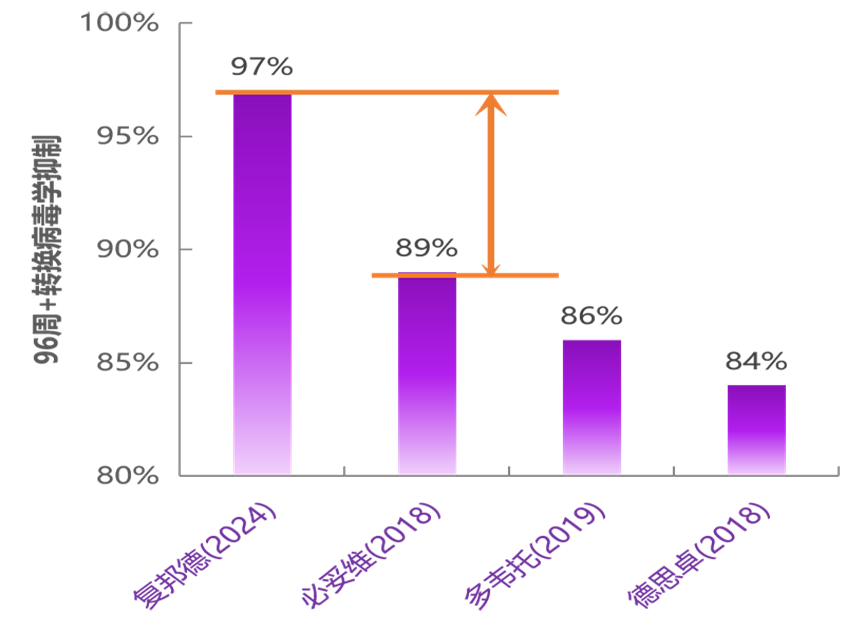
*Based on statistics from published research results
This regimen is the only antiretroviral therapy in clinical trials to date that has surpassed the 95% viral suppression target set by UNAIDS [5].
· Significant Advantage in Immune Reconstitution:
At Week 96, CD4+ cell counts continued to increase similarly in both groups from Week 48 (ISG vs. DSG, 28.0 [95% CI: 12.0, 44.0] vs. 31.5 [16.1, 47.0] cells/μL).
· Sustained Safety Profile:
Overall incidence of adverse events was low, with serious adverse events being rare. The DSG regimen provided additional metabolic benefits, including a slight decrease in body weight (average -0.59 kg) and significant improvement in LDL-C (-0.30 mmol/L from Week 48 to Week 96), while levels remained largely stable in the ISG (change of 0.01 mmol/L). Other metabolic and safety indicators, such as blood glucose and renal function, also remained stable.
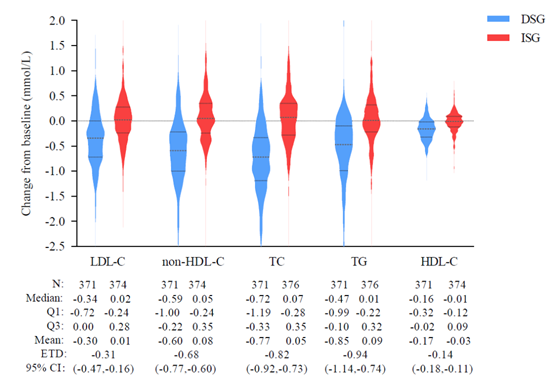
*Violin Plot of Lipid Profile Changes from Week 48 to Week 96 in the SPRINT Study
Rooted in China, Benefiting the World: Providing a Improved Long-Term Management Strategy for PWH
The academic rigor and clinical value of the 96-week SPRINT data have been highly recognized by international peer-review experts.
Prof. Assoumou Lambert
French National Institute of Health and Medical Research (INSERM)
In the context of chronic HIV management, optimizing ARV regimens no longer focuses solely on viral suppression; reducing metabolic risk is equally important. This study demonstrates that Ainuomiti Tablets maintained excellent virological efficacy (>96% suppression) at 96 weeks, confirming its non-inferiority compared with the control regimen. The metabolic results are particularly encouraging: moderate weight loss and improved lipid profile were observed in the DSG. This new therapy is beneficial for the long-term cardiometabolic management of the HIV population.
Prof. Diego Ripamonti
Pope John XXIII Hospital, Bergamo, Italy
This study enrolled 757 PWH. Following the extension phase, virological efficacy was comparable between groups, with over 97% of PWH in each group achieving plasma HIV RNA <50 copies/mL. Notably, switching from E/C/F/TAF to Ainuomiti Tablets showed beneficial changes in body weight and lipid improvements. Overall, serious adverse events were very rare. This study provides important evidence for expanding HIV treatment strategy options and has significant clinical implications.
Currently, besides being approved and marketed in China, Aitribond® is either registered or under review for marketing authorization in several African countries. The publication of the SPRINT 96-week data not only signifies international academic recognition for this China-originated anti-HIV regimen but also means that countless PWH on antiretroviral therapy may directly benefit from these findings. Particularly for those facing metabolic challenges such as weight gain and dyslipidemia due to long-term medication, Aitribond® offers a treatment pathway characterized by high efficacy and metabolic friendliness, demonstrating significant clinical advantages.

