French Local Time, October 15-18, the 20th European AIDS Conference (EACS) was held in Paris. During the conference's "Forever Three-in-One" session, Professor Li Linghua from Guangzhou Medical University's Affiliated Eighth Hospital was invited to deliver an oral presentation, systematically introducing the 144-week SPRINT study results of Aidea Pharma's first self-developed innovative anti-HIV single-tablet regimen Aitribond® (ALT) in switch therapy. This marks the first time a Chinese-developed innovative anti-HIV drug has been featured in a dedicated oral presentation at a mainstream HIV academic conference in western countries.
Figure 1. Professor Li Linghua presenting the study at the European AIDS Conference
As a specialized HIV conference with extensive international influence, EACS attracts global HIV prevention and treatment experts to participate and share cutting-edge advances and practical experiences.
Currently, although second-generation integrase inhibitor regimens are widely used in clinical practice, significant unmet clinical needs remain in areas such as emerging drug resistance, cardiometabolic complications, and drug-drug interactions. Against this backdrop, the clinical research results on Aitribond® released this time have attracted significant attention.
01 Chinese-made anti-HIV New Drug Presented at Mainstream Conference in western countries
Study data show that Aitribond® demonstrates sustained clinical benefits and reliable safety in long-term treatment. Among the subjects who entered the extension phase, the vast majority completed 144 weeks of treatment, with good overall treatment adherence. Virologically, both people living with HIV(PWH) groups achieved high viral suppression rates, and CD4+ T-cell counts showed a steady upward trend, indicating durable immune recovery capacity [1].
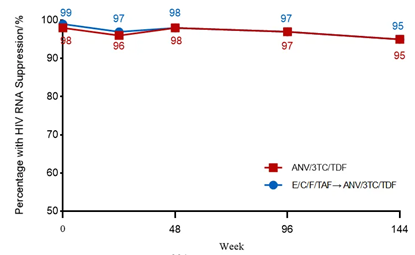
Figure 2. Viral suppression in HIV-1 infected PWH over 144 weeks after switching therapy
In terms of metabolic safety, Aitribond® demonstrated positive impacts. Fasting low-density lipoprotein cholesterol (LDL-C) levels of PWH continued to improve, with more pronounced improvement particularly in PWH switching from the E/C/F/TAF regimen; the proportion of PWH with high-risk LDL-C decreased in both groups. Furthermore, after 144 weeks of treatment, changes in estimated glomerular filtration rate (eGFR) of PWH aligned with the natural trend of aging, suggesting a favorable renal safety profile for the drug [1].
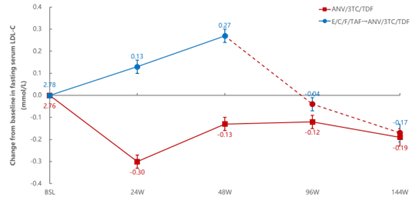
Figure 3. Change from baseline in fasting serum LDL-C over 144 weeks
02 Multiple Studies Expand the Clinical Evidence System for Aitribond®
The conference also presented seven other studies on Aitribond® in poster format. Regarding comprehensive metabolic benefits: research confirmed that switching to Aitribond® helps improve dyslipidemia and promotes the return of multiple lipid parameters to ideal ranges.
In analyses of special populations, Aitribond® also performed excellently: In PWH with pre-existing NNRTI resistance, its virological suppression efficacy was non-inferior to the control group; in female PWH, it maintained viral suppression while improving lipid metabolism and reducing neuropsychiatric adverse events; in young PWH, it demonstrated better CD4 cell recovery, less weight gain, and improved lipid control.
Long-term safety pooled analysis further indicated: Aitribond® maintained stable renal function indicators throughout the 144-week treatment period and showed sustained advantages in improving insulin sensitivity and regulating blood lipids [2-8].
These solid research data collectively build the unique value system of Aitribond®: its single-tablet regimen ensures durable viral suppression while significantly improving lipid metabolism and optimizing weight management without additional medications; leveraging the price advantage brought by Chinese innovation, it substantially enhances medication accessibility; it fills the market gap for metabolism-friendly antiviral drugs in China, contributing to public health prevention and treatment goals through its treatment convenience and cost-effectiveness. Based on these advantages, Aitribond® demonstrates outstanding clinical value and public health value.

[1] Long-term viral suppression, and cardiometabolic benefits of switch to ainuovirine coformulated with lamivudine, and tenofovir DF in virologically suppressed people with HIV-1: 144-week, open-label results from the SPRINT post-study.
[2] Improved atherosclerotic cardiovascular disease risk-associated dyslipidemia strata in people with HIV-1 switching to ainuovirine-based antiretroviral regimen: week 144 results from SPRINT, a randomized phase 3 trial.
[3] High virologic suppression in treatment-naïve people with HIV-1 on ainuovirine- versus efavirenz-based antiretroviral regimen as initial therapy: effect of pretreatment NNRTI resistance from RACER trial, a multi-center, randomized, active-controlled study.
[4] Efficacy, and safety of ainuovirine-based antiretroviral regimen in women with HIV-1: the pooled analysis of the RACER, and SPRINT studies, two multicenter, randomized, active controlled phase 3 trials.
[5] Efficacy, and safety of ainuovirine plus lamivudine, and tenofovir DF in young people with HIV-1: pooled analyses of RACER, and SPRINT studies, two randomized, active-controlled phase 3 trials.
[6] Preserved renal function in PWH on ainuovirine-based antiretroviral regimen: week 144 results from SPRINT study, a randomized phase 3 trial.
[7] Long-term changes in triglyceride-glucose index and insulin resistance in virologically suppressed people with HIV-1 switching to ainuovirine coformulated with lamivudine, and tenofovir DF: 144-week, open-label results from the SPRINT post-study.
[8] Long-term Lipid Metabolism Benefits of Switching to Ainuovirine-based Regimen in People with HIV-1: 144-Week Results from the SPRINT Extensional Post-study.

