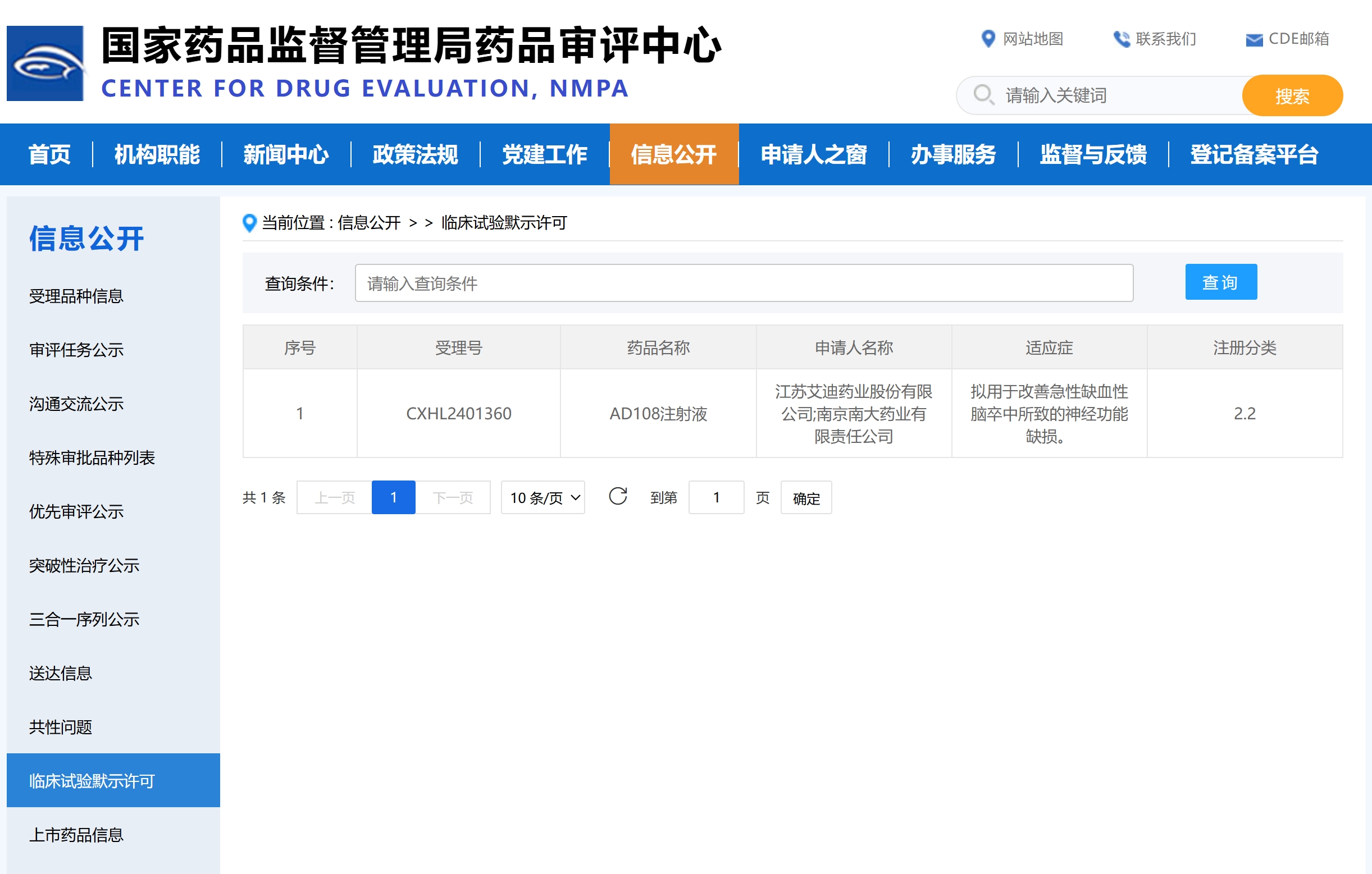
On March 12, 2025, Jiangsu Aidea Pharmaceutical Co., Ltd. (hereinafter referred to as "Aidea Pharma") and its holding subsidiary, Nanjing Nanda Pharmaceutical Co., Ltd. (hereinafter referred to as "Nanda Pharma"), received the Drug Clinical Trial Approval Notice issued by the National Medical Products Administration (NMPA), granting approval to initiate Phase I clinical trials for AD108 Injection, a Category 2.2 new drug. The drug is intended to improve neurological deficits caused by acute ischemic stroke (AIS).
AD108 Injection is an improved new drug with the active ingredient human urinary kallidinogenase, also known as tissue kallikrein-1 (KLK-1). KLK-1 is a serine protease secreted by the kidneys, exerting its biological effects through the kallikrein-kinin system (KKS). The KKS interacts with and counterbalances the renin-angiotensin system (RAS), together playing a crucial role in maintaining the stability and functionality of the circulatory system. Extensive medical research has demonstrated that impaired KKS function is a critical contributor to the onset, progression, and poor prognosis of cardiovascular and cerebrovascular diseases, making it a natural therapeutic target for conditions such as stroke.
Evidence-based medical studies have demonstrated that exogenous supplementation of KLK-1 can effectively improve neurological deficits in AIS patients, reduce disability rates, and enhance daily living capabilities. AD108 Injection achieves sustained therapeutic effects by maintaining stable plasma concentrations through subcutaneous administration, thereby avoiding the risk of blood pressure drops associated with intravenous delivery.
The development of AD108 Injection aims to provide a more effective treatment option for AIS patients, demonstrating immense market potential.
The approval of this IND application serves as a significant recognition of Aidea Pharma and Nanda Pharma’s R&D capabilities and innovation strength. Moving forward, the two parties will deepen their collaboration to accelerate the clinical trial progress of AD108 Injection, striving to bring this therapy to market at the earliest opportunity. This will provide patients with enhanced treatment options while creating new growth opportunities for the company. Furthermore, both entities will optimize the allocation and integration of R&D, production, and commercial resources, expedite the deployment and execution of their R&D pipeline, and continuously strengthen their core competitiveness in the pharmaceutical sector to deliver greater value to patients and shareholders.
About Aidea Pharma
Jiangsu Aidea Pharmaceutical Co., Ltd. (“Aidea Pharma”, 688488.SH) was established in 2009 and listed on the Shanghai Stock Exchange STAR Market in 2020. As a high-tech pharmaceutical company, Aidea Pharma focuses on developing and marketing comprehensive anti-HIV/AIDS product portfolios while leveraging its competitive advantages in the research and production of human urine-derived proteins.
About Nanda Pharma
Nanjing Nanda Pharmaceutical Co., Ltd. (“Nanda Pharma”), established in 1998 in Nanjing, is a holding subsidiary of Aidea Pharma. Nanda Pharma specializes in the research, development, production, and sales of biochemical active pharmaceutical ingredients (APIs) and formulations.

