On 20th July, the Phase III SPRINT study of Aitribond® (Ainuovirine, Lamivudine and Tenofovir Disoproxil Fumarate Tablets, ANV/3TC/TDF), an oral anti-HIV new drug independently developed by Jiangsu Aidea Pharmaceutical Co., Ltd. (hereinafter referred to as Aidea Pharma), was published in full online in The Lancet Regional Health - Western Pacific, an authoritative international medical journal.
Aitribond® is a fixed-dose combination based on the oral anti-HIV new drug, Ainuovirine, combined with lamivudine and tenofovir disoproxil fumarate. It is the first innovative oral anti-HIV three-in-one single-tablet regimen with independent intellectual property rights in China. Ainuovirine Tablets is a new generation of non-nucleoside reverse transcriptase inhibitor (NNRTI) independently developed by Aidea Pharma. It has non-inferior efficacy compared to Efavirenz (EFV), with lower rates of neuropsychiatric symptoms, liver toxicity and dyslipidemia associated with the classic NNRTI. In 2021, it has been approved by the National Medical Products Administration and recommended as the first-line treatment regimen by Chinese clinical guidelines.
Previously, the Phase III clinical trial of Ainuovirine Tablets (RACER study, Research of Efficacy of Ainuovirine- Controlled by Efavirenz-Based Regimen) was published in The Lancet Regional Health - Western Pacific on 24th April, 2023. Aitribond® were once again presented in this top international clinical medicine journal, which not only filled the gap in evidence-based medicine field of anti-HIV treatment in China, but also made a significant contribution to the anti-HIV cause in the Western Pacific region.

The SPRINT study is a multi-center, randomized, double-blind, double-dummy, active-controlled phase III clinical trial led by Professor Fujie Zhang from Beijing Ditan Hospital affiliated to Capital Medical University. A total of 10 clinical centers across China were involved, and a total of 762 people with HIV (PWH) were enrolled. It is the first international randomized controlled trial (RCT) to directly compare efficacy and safety outcomes on switching treatment with NNRTI- and INSTI- based regimen in Chinese population. The primary efficacy endpoint is the percentage of subjects with HIV-1 RNA levels ≥50 copies/mL in the test group (ANV/3TC/TDF) not inferior to that in the control group (Elvitegravir, Cobicistat, Emtricitabine and Tenofovir Alafenamide Fumarate Tablets, EVG/Cobi/FTC/TAF) at week 48, with the non-inferiority margin of 4%. After completing 48-week double-blind treatment, a 48-week open-label study began, in which the treatment regimen of all subjects was switched to ANV/3TC/TDF.
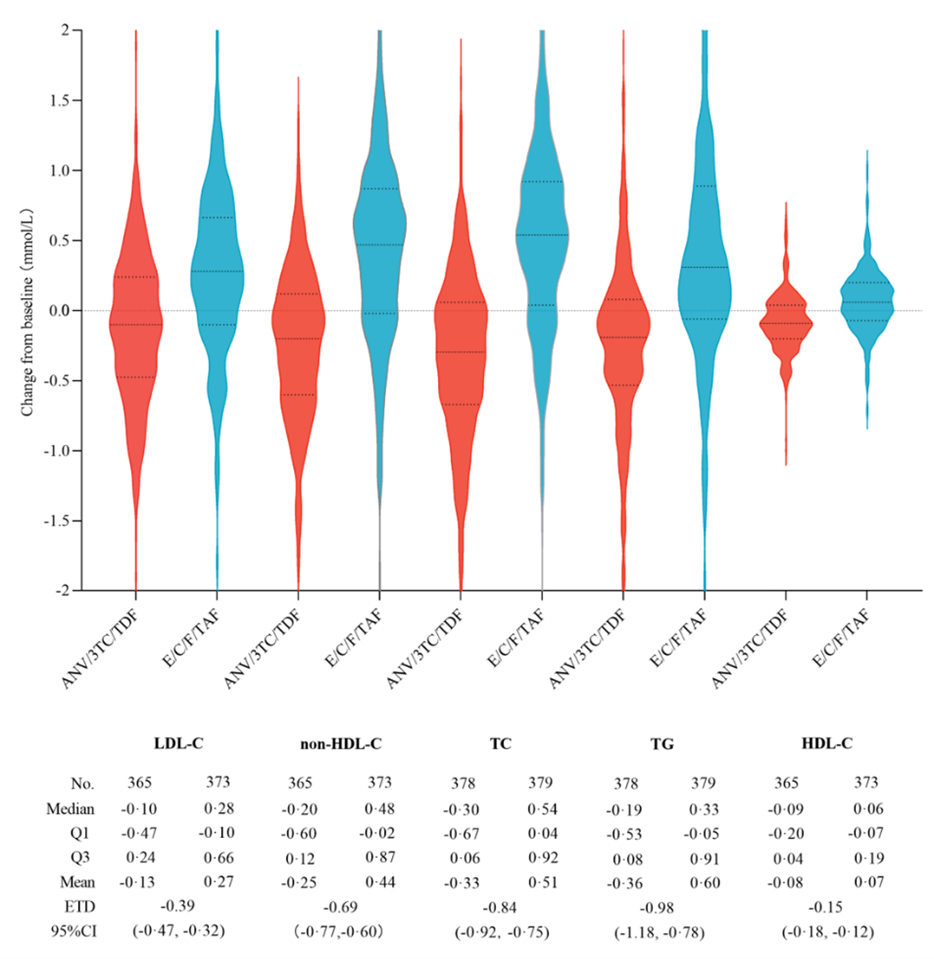
At week 48, 7 (1.8%) participants on ANV/3TC/TDF and 6 (1.6%) participants on EVG/Cobi/FTC/TAF had plasma HIV-1 RNA titer at 50 copies per mL or above including missing virological data within the time window (the Cochran-Mantel-Haenszel method, estimated treatment difference [ETD], 0.3%, 95%CI-1.6 to 2.1), establishing the non-inferiority of ANV/3TC/TDF to EVG/Cobi/FTC/TAF. At week 48, the participants on ANV/3TC/TDF showed a significantly less weight gain from baseline compared to those on EVG/Cobi/FTC/TAF (least square mean, 1.16 versus 2.05 kg, -0.90 kg, -1.43 to -0.37). The changes in blood lipid profile from baseline showed that ANV/3TC/TDF was friendlier than EVG/Cobi/FTC/TAF, with low-density lipoprotein cholesterol (LDL-C) ETD of -0.39 mmol per L [-0.47, -0.32]. This study showed that in virologically suppressed PWH, switching to ANV/3TC/TDF can maintain virological suppression non-inferior to that of EVG/Cobi/FTC/TAF, and has benefits in terms of cardiovascular metabolic safety such as blood lipid metabolism.
It is worth mentioning that, the Joint United Nations Programme on HIV/AIDS (UNAIDS) and the World Health Organization (WHO) reiterated at the International AIDS Conference 2024 in Munich, Germany, that the fourth "95" goal should be achieved on the basis of the "95-95-95" target, that is, 95% of PWH should have a higher quality of life. The Phase III SPRINT study of switching treatment with Aitribond® confirmed that ANV/3TC/TDF can improve cardiovascular metabolic risks such as weight gain and dyslipidemia compared with the first-line INSTI- based regimen, forming a good complement to the first-line regimen, and can provide better medical care for PWH.
In addition, during the pre-IND communication with the US FDA, the US FDA expressed high recognition to the clinical value and quality of the Phase III RACER study in treatment-naïve population and the phase III SPRINT study in treatment-experienced population conducted by Aidea Pharma. The dual recognition of Ainuomiti Tablets (Aitribond®) and Ainuovirine Tablets by professional academic journals and high-level regulatory authorities is a solid foundation for Aidea Pharma to further expand its presence in the international market.

