
Jiangsu Aidea Pharmaceutical Co., Ltd. (referred to as Aidea Pharma) will present the findings from three clinical studies on the new drug Ainuovirine (ANV) Tablets and its three-in-one combination Ainuomiti Tablets (ANV/3TC/TDF) at the 9th Asia-Pacific AIDS and Co-infections Conference (APACC 2024).
Recognized as the most influential HIV/AIDS academic conference in the Asia-Pacific region, APACC 2024 will be held in Hong Kong, China, from June 27 to 29, 2024. It provides a vital scientific platform focusing on the developments, challenges, and needs in the field of AIDS in the Asia-Pacific region. Researchers have the opportunity to present and discuss the latest developments and are offered opportunities for interaction, networking, and building collaborations.
Ainuovirine Tablets, approved for marketing in June 2021, is the first domestically developed oral anti-HIV/AIDS new drug in China. As a new generation of non-nucleoside reverse transcriptase inhibitors (NNRTIs), Ainuovirine Tablets has excellent pharmacological characteristics such as strong antiviral activity, less cross-resistance, high target selectivity, and fewer drug-drug interactions.
Ainuomiti Tablets, a combination of Ainuovirine with Lamivudine and Tenofovir Disoproxil Fumarate, is the first independently developed anti-HIV/AIDS innovative single-tablet combination in China.
In particular, Professor Fujie Zhang from Beijing Ditan Hospital Affiliated to Capital Medical University will be invited to give an oral presentation on the 48-week results of the Phase III clinical trial of Ainuomiti Tablets (SPRINT study) in treatment-experienced people living with HIV (PLWH) on June 28, 2024, indicating the scientific impact of Ainuomiti Tablets and SPRINT study in the Asia-Pacific region.
The SPRINT study is a multicenter, randomized, double-blind, double-dummy, active-controlled, non-inferiority study to evaluate the efficacy and safety of Ainuomiti Tablets versus Genvoya® (EVG/Cobi/FTC/TAF) in virologically suppressed PLWH [1]. A total of 762 treatment-experienced adults with HIV were randomly assigned (1:1) to receive ANV/3TC/TDF or EVG/Cobi/FTC/TAF for up to 48 successive weeks, followed by an optional open-label extension period with 48-week ANV/3TC/TDF treatment. The 48-week study data will be presented at this conference. This study showed that in virologically suppressed PLWH, switch to ANV/3TC/TDF can maintain virological suppression non-inferior to that to EVG/Cobi/FTC/TAF, and has benefits in terms of cardiovascular metabolic safety such as blood lipid metabolism.

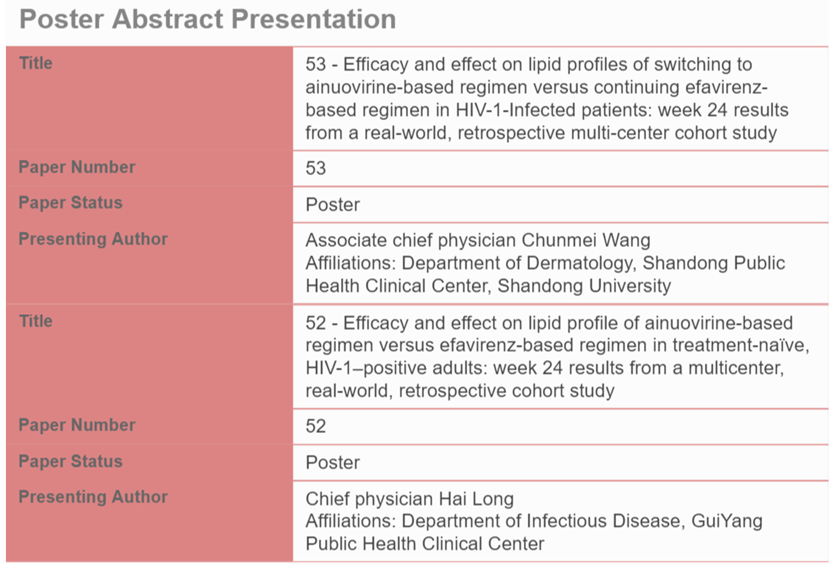
In addition, two post-marketing real-world studies on Ainuovirine Tablets will be also presented by Professor Hai Long from Guiyang Public Health Clinical Center, and Professor Chunmei Wang from Shandong Public Health Clinical Center, respectively.
Post-Marketing Real-World Study 1: It is a multicenter, real-world, retrospective cohort study conducted in 6 cities in China. Data from 274 eligible PLWH on ANV-based regimen (ANV/3TC/TDF) and 541 PLWH on EFV-based regimen (EFV+3TC+TDF) were collected and analyzed. Results indicated that the ANV-based regimen was well tolerated in treatment-naïve PLWH with effective HIV-RNA suppressing effect, improved CD4+ cell counts, and fewer lipid abnormalities.
Post-Marketing Real-World Study 2: Another multi-center, retrospective, controlled cohort study compared the lipid profile changes in treatment-experienced PLWH switching to the ANV-based regimen from EFV-based regimen [3]. A total of 318 participants were eligible for the ANV group and 460 participants for the EFV group. The study found good efficacy, and favorable changes in lipid profile of treatment-experienced PLWH switching from EFV-based regimen to ANV-based regimen, providing a beneficial switch option for those at risk of metabolic syndrome or cardiovascular diseases.
The presentation of Aidea Pharma’s clinical research findings on Ainuovirine Tablets and its single-tablet combination Ainuomiti Tablets at APACC 2024 is extraordinary significant. This is not only the recognition of the scientific and regulatory compliance of China’s clinical research on innovative anti-HIV/AIDS drugs by the Asia-Pacific anti-HIV/AIDS academic community, but also marks the beginning of Aidea Pharma's entry into the Asia-Pacific market. Aidea Pharma and lead researchers will share these findings and discuss the further development of anti-HIV/AIDS new drugs with the top experts in the context of the Asia-Pacific HIV epidemiology, to promote the advanced antiretroviral drugs from China to go abroad and benefit HIV patients across the region.
Reference
[1] Zhang F, Wu H, Ma P, et al. Noninferior efficacy, reduced weight gain, and improved lipid metabolism of switch to ainuovirine- versus boosted elvitegravir- based regimen in virologically suppressed people living with human immunodeficient virus 1: the 48-week results of the SPRINT trial [C]. Asia Pacific AIDs & Co-Infections Conference, 2024.
[2] Long H, He Q, Bi Y, et al. Efficacy and effect on lipid profile of ainuovirine-based regimen versus efavirenz-based regimen in treatment-naïve, HIV-1-positive adults: week 24 results from a multicenter, real-world, retrospective cohort study[C]. Asia Pacific AIDs & Co-Infections Conference, 2024.
[3] Wang C, Yu X, Ke Y, et al. Efficacy and effect on lipid profiles of switching to ainuovirine-based regimen versus continuing efavirenz-based regimen in HIV-1-Infected patients: week 24 results from a real-world, retrospective multi-center cohort study [C]. Asia Pacific AIDs & Co-Infections Conference, 2024.
About Ainuovirine Tablets and Ainuomiti Tablets
Ainuovirine Tablets was approved for marketing in June 2021 and is the first original oral new drug for the HIV-1 treatment in China. In the same year, Ainuovirine Tablets was officially recommended in Chinese Guidelines for the Diagnosis and Treatment of HIV/AIDS (2021 Edition). Ainuomiti Tablets, a combination of ainuovirine, lamivudine and tenofovir disoproxil fumarate, was approved for marketing in December 2022 and is the first three-in-one single-tablet combination anti-HIV/AIDS new drug with independent intellectual property rights in China.
About SPRINT Study on Ainuomiti Tablets
The SPRINT study is a multicenter, randomized, double-blind, double-dummy, active-controlled, non-inferiority study to evaluate the efficacy and safety of Ainuomiti Tablets (ANV/3TC/TDF) versus Genvoya® (EVG/Cobi/FTC/TAF) in virologically suppressed PLWH. A total of 762 participants who had been treated with a NNRTI combined with two NRTIs for at least 12 months were randomly assigned (1:1) to receive Ainuomiti Tablets or Genvoya® for up to 48 successive weeks, followed by an optional open-label extension period with 48-week Ainuomiti treatment.
About Adiea Pharma
Aidea Pharma is an innovative pharmaceutical company dedicated to the R&D of new chemical drugs and human urine-derived protein products. At present, its core pipeline includes eight Class I new drugs and four Class 2 new drugs, with anti-HIV/AIDS regimens including NNRTIs, integrase inhibitors and long-acting therapeutic drugs, and treatment for inflammation and stroke. To date, Aidea Pharma has been granted a total of 30 patents.
The two innovative drugs, Ainuovirine Tablets and Ainuomiti Tablets, have been approved for marketing in the past few years in China. ACC017, an Integrase strand transfer inhibitor with a new chemical structure, was approved for drug clinical trials in January 2024 and has moved to the Phase I clinical trial stage. ACC027, a long-acting drug for HIV treatment, is also under the development. Through the layout and development of a series of antiviral new drug pipelines, Aidea Pharma strives to provide more comprehensive and diverse options for PLWH, and continuously meet the urgent needs of HIV/AIDS treatment upgrading.
Disclaimer
The above content is compiled from publicly published papers and does not represent the views of Aidea Pharma. It is only for the purpose of medical professionals learning and communication, and does not constitute commercial promotion or treatment advice. If you need treatment, please contact a professional physician.

