In order to promote the scientific development of AIDS and STD prevention and control in China, exchange and share research progress and experience in prevention and treatment, the 8th National Academic Conference on HIV/AIDS was held by Chinese Association of STD and AIDS Prevention and Control (CASAPC) on April 10-13th in Xiamen, Fujian Province, on the theme of "Fighting HIV/AIDS and Sharing Health". As the first innovative drug company to participate in the "Collection of Real-World Data (RWD) on HIV/AIDS Clinical Diagnosis and Treatment" project launched by the CASAPC, Jiangsu Aidea Pharmaceutical Co., Ltd. (referred to as Aidea Pharma) was invited to attend and delivered a speech. At the meeting, Aidea Pharma shared its efforts on HIV/AIDS prevention and control, as well as the achievements and long-term significance of the HIV/AIDS iStudy Program since its execution.

Sponsored by the CASAPC, this conference has become the grandest HIV/AIDS academic communication event in China since its first session in 2014. The conference invited experts and scholars in the field of HIV/AIDS prevention and control at home and abroad to exchange and share the latest information, achievements and progress of AIDS/STD and related diseases prevention and control in China and the world. In addition, special activities such as main venue reports, sub-forums and thematic forums, satellite conferences, poster exchanges, exhibitions and others were held during the conference. Among them, the HIV/AIDS iStudy Program Forum is the first agenda of the conference.
The HIV/AIDS iStudy Program is a cooperative project between CASAPC and Aidea Pharma based on the collection of RWD on HIV/AIDS clinical diagnosis and treatment. The two sides have carried out close cooperation in exploring innovative HIV/AIDS treatment regimens, the establishment of an integrated management model for diagnosis and treatment, and strengthening the capacity of HIV/AIDS practitioners. The HIV/AIDS Research Program involved the innovative oral anti-HIV new drug, Ainuovirin, independently developed by Aidea Pharma, into the structured real-world database to build China's own HIV/AIDS real-world database. By the end of 2022, nearly 7,000 patients have been enrolled in the Ainuovirine treatment regimen; covering 29 provinces/autonomous regions and municipalities across China, and nearly 40 clinical centers and 267 HIV/AIDS prevention and control institutions at all levels have participated. It shows a good development trend with wide coverage, many participating units and great influence. This will lay the foundation for the follow-up studies related to the Ainuovirine regimen, and also provide evidence-based medical basis for the formulation of medical and public health measures.

At the meeting, Dr. Heliang Fu, the chairman of Aidea Pharma, delivered a speech on "Reviewing the HIV/AIDS iStudy Program from the Perspective of Aidea Pharma", and Ms. Yufang Zheng on the theme of "The Output of Medical Big Data Results from HIV/AIDS iStudy Program", sharing the anti-HIV/AIDS achievements from the perspective of the enterprise. Dr. Fu said, "The HIV/AIDS iStudy Program has provided a unique industry academic platform to interact with experts and doctors at all levels, with the influence of experts at home and abroad, exerting the capabilities of young and middle-aged practitioners as well as the grassroots from the front-line, sharing the most cutting-edge academic information in the field to improve the scientific research level and discipline influence of medical workers, and jointly build an evidence-based medical research platform for new anti-HIV drugs development. It is conducive to establish a long-term and reliable cooperative relationship between Aidea Pharma and HIV/AIDS diagnosis and treatment community, and build a Chinese regimen of HIV/AIDS treatment with evidence-based medical basis that is more effective, safer, more convenient, with higher compliance, and suitable for China's national conditions."
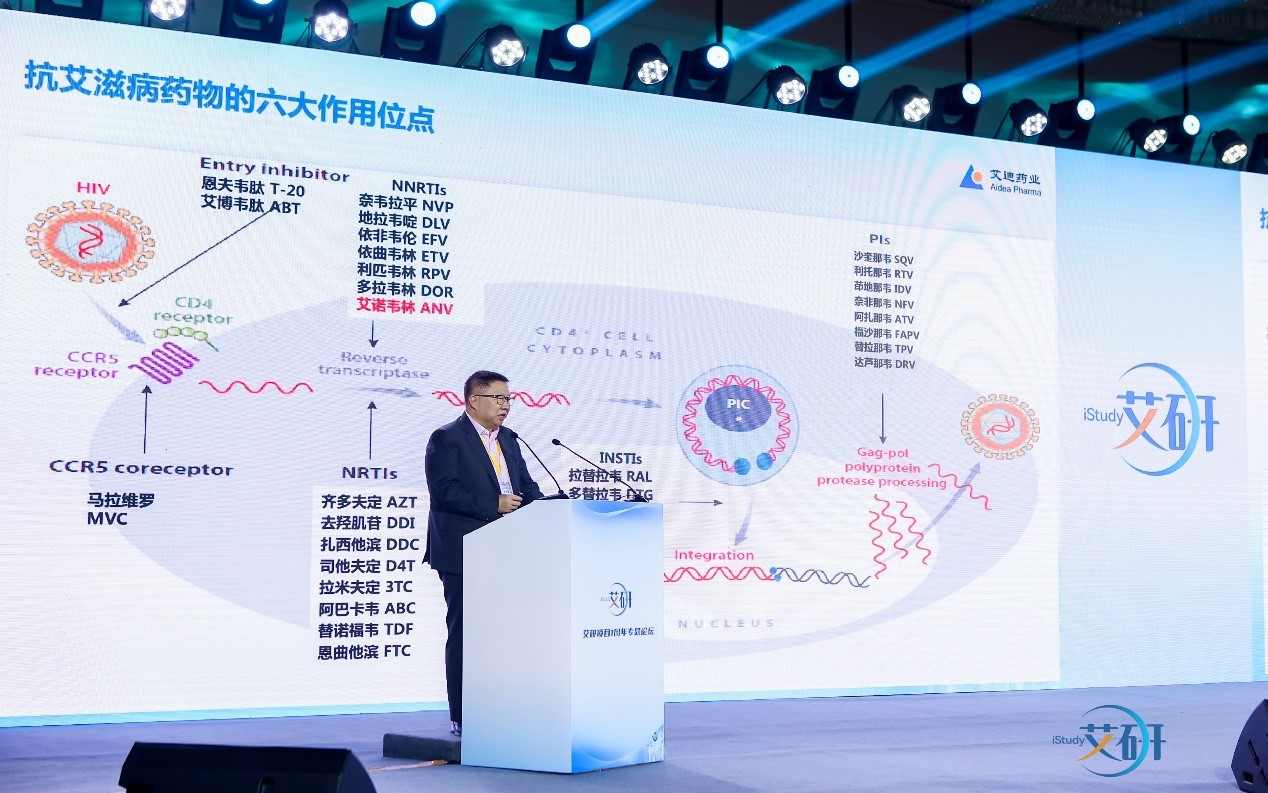
Aidea Pharma currently has two new anti-HIV drugs with independent intellectual property rights. In 2021, Ainuovirine tablet was approved, and was included in the List for National Basic Medical Insurance in December of the same year. Subsequently, the three-in-one compound formulation, Ainuomiti tablet, with ainuovirine as the core ingredient, was approved on December 30th, 2022, forming a complete antiviral treatment regimen. That provides a new alternative in line with international treatment regimen for HIV-infected individuals in China, and meet the growing demand for diagnosis and treatment of domestic HIV infectors. During the process of new drug development, the two products were recognized by the Drug Evaluation Center of the NMPA, and were successively included in the National Major Scientific and Technological Special Project for "Significant New Drugs Development” of China during the 13th Five-Year Plan period.
The struggle between human beings and HIV/AIDS is still going on. In the future, HIV/AIDS prevention and control will face new challenges and new situations. Aidea Pharma will adhere to the concept of "Leading Innovation and Dreams ", aim at the major diseases areas that seriously threaten human health, such as HIV/AIDS, inflammation, stroke, and condense the wisdom and achievements of HIV/AIDS prevention and treatment, to provide high-quality innovative drugs to patients and healthcare workers worldwide.

